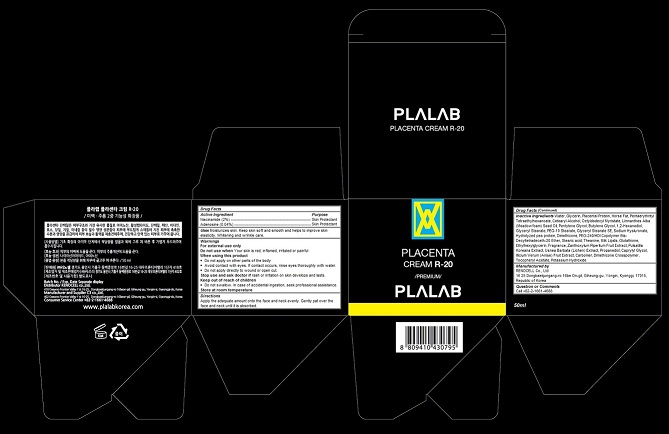
Label: PLALAB PLACENTA R-20- niacinamide, adenosine cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 70326-103-01, 70326-103-02 - Packager: RENOCELL Co., Ltd
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 23, 2015
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Niacinamide (2%)Adenosine (0.04%)
- Skin Protectant
- Keep out of reach of children Do not swallow. In case of accidental ingestion, seek professional assistance.
- Moisturizes skin. Keep skin soft and smooth and helps to improve skin elasticity. Whitening and wrinkle care.
- WarningsFor external use onlyDo not use when Your skin is red, inflamed, irritated or painfulWhen using this product Do not apply on other parts of the body Avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water. Do not apply directly to wound or open cut.Stop use and ask doctor if rash or irritation on skin develops and lasts.Store at room temperature
- DirectionsApply the adequate amount onto the face and neck evenly. Gently pat over the face and neck until it is absorbed.
- Water, Glycerin, Placental Protein, Horse Fat, Pentaerythrityl Tetraethylhexanoate, Cetearyl Alcohol, Octyldodecyl Myristate, Limnanthes Alba (Meadowfoam) Seed Oil, Pentylene Glycol, Butylene Glycol, 1,2-Hexanediol, Glyceryl Stearate, PEG-10 Stearate, Glyceryl Stearate SE, Sodium Hyaluronate, Hydrolyzed pea protein, Dimethicone, PEG-240/HDI Copolymer Bis-Decyltetradeceth-20 Ether, Stearic acid, Theanine, Milk Lipids, Glutathione, Ethylhexylglycerin, Fragrance, Zanthoxylum Piperitum Fruit Extract, Pulsatilla Koreana Extract, Usnea Barbata (Lichen) Extract, Propanediol, Caprylyl Glycol, Illicium Verum (Anise) Fruit Extract, Carbomer, Dimethicone Crosspolymer, Tocopheryl Acetate, Potassium Hydroxide
- Plalab Placenta Cream R-20
-
INGREDIENTS AND APPEARANCE
PLALAB PLACENTA R-20
niacinamide, adenosine creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70326-103 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 0.02 in 50 mL ADENOSINE (UNII: K72T3FS567) (ADENOSINE - UNII:K72T3FS567) ADENOSINE 0.0004 in 50 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) SUS SCROFA PLACENTA (UNII: C8CV8867O8) Glycerin (UNII: PDC6A3C0OX) Pentaerythrityl Tetraethylhexanoate (UNII: XJ7052W897) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) Octyldodecyl Myristate (UNII: S013N99GR8) MEADOWFOAM SEED OIL (UNII: 412ZHA4T4Y) Pentylene Glycol (UNII: 50C1307PZG) Butylene Glycol (UNII: 3XUS85K0RA) 1,2-Hexanediol (UNII: TR046Y3K1G) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-10 Stearate (UNII: D3AHD468TV) Glyceryl Stearate SE (UNII: FCZ5MH785I) HYALURONATE SODIUM (UNII: YSE9PPT4TH) HYDROLYZED SOY PROTEIN (ENZYMATIC; 2000 MW) (UNII: 1394NXB9L6) Dimethicone (UNII: 92RU3N3Y1O) Stearic acid (UNII: 4ELV7Z65AP) Theanine (UNII: 8021PR16QO) COW MILK FAT (UNII: 463JZS0XJ3) Glutathione (UNII: GAN16C9B8O) Ethylhexylglycerin (UNII: 147D247K3P) ZANTHOXYLUM PIPERITUM FRUIT PULP (UNII: 7PFC2VA251) PULSATILLA KOREANA ROOT (UNII: FY35I16MPL) USNEA BARBATA (UNII: D6DVA9TCAP) Propanediol (UNII: 5965N8W85T) Caprylyl Glycol (UNII: 00YIU5438U) STAR ANISE FRUIT (UNII: CK15HA8438) CARBOXYPOLYMETHYLENE (UNII: 0A5MM307FC) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70326-103-02 1 in 1 PACKAGE 1 NDC:70326-103-01 50 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 12/23/2015 Labeler - RENOCELL Co., Ltd (689605795) Registrant - RENOCELL Co., Ltd (689605795) Establishment Name Address ID/FEI Business Operations RENOCELL Co., Ltd 689605795 manufacture(70326-103)