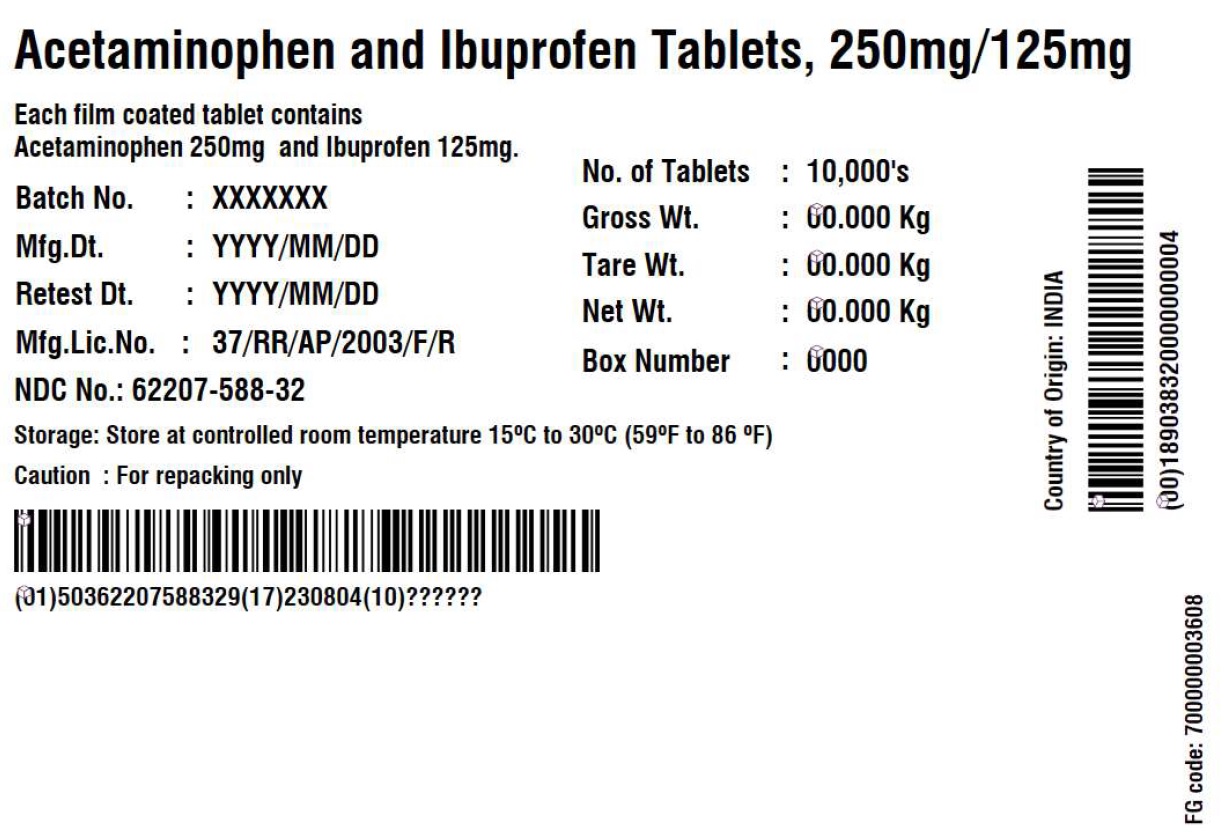
Label: ACETAMINOPHEN AND IBUPROFEN tablet
- NDC Code(s): 62207-588-32, 62207-588-85
- Packager: Granules India Limited
- Category: BULK INGREDIENT
- DEA Schedule: None
- Marketing Status: Export only
Drug Label Information
Updated July 10, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ACETAMINOPHEN AND IBUPROFEN
acetaminophen and ibuprofen tabletProduct Information Product Type BULK INGREDIENT Item Code (Source) NDC:62207-588 Route of Administration NOT APPLICABLE Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 250 mg IBUPROFEN (UNII: WK2XYI10QM) (IBUPROFEN - UNII:WK2XYI10QM) IBUPROFEN 125 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) HYPROMELLOSE 2910 (15 MPA.S) (UNII: 36SFW2JZ0W) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYDEXTROSE (UNII: VH2XOU12IE) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POVIDONE K30 (UNII: U725QWY32X) POVIDONE K90 (UNII: RDH86HJV5Z) CARNAUBA WAX (UNII: R12CBM0EIZ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) CROSPOVIDONE (UNII: 2S7830E561) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HYPROMELLOSE 2910 (3 MPA.S) (UNII: 0VUT3PMY82) STARCH, CORN (UNII: O8232NY3SJ) SODIUM LAURYL SULFATE (UNII: 368GB5141J) STEARIC ACID (UNII: 4ELV7Z65AP) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color yellow (Light yellow to yellow colored) Score no score Shape CAPSULE (capsule shaped, biconvex) Size 14mm Flavor Imprint Code G;131 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62207-588-32 10000 in 1 DRUM 08/30/2023 2 NDC:62207-588-85 90000 in 1 DRUM 03/01/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 08/30/2023 Labeler - Granules India Limited (915000087) Establishment Name Address ID/FEI Business Operations Granules India Limited 918609236 analysis(62207-588) , manufacture(62207-588)