Label: LONGRANGE- eprinomectin injection, solution
- NDC Code(s): 0010-7216-02, 0010-7216-03, 0010-7216-04
- Packager: Boehringer Ingelheim Animal Health USA Inc.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated December 20, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
VETERINARY INDICATIONS
5% Sterile Solution
Extended-Release Injectable
For Subcutaneous Use Parasiticide for Cattle on Pasture
For the Treatment and Control of Internal and External Parasites of Cattle on Pasture with Persistent Effectiveness
Not for use in female dairy cattle 20 months of age or older, including dry dairy cows. Not for use in calves to be processed for veal.
Not for use in breeding bulls, or in calves less than 3 months of age.
Not for use in cattle managed in feedlots or under intensive rotational grazing.
Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
-
DESCRIPTION
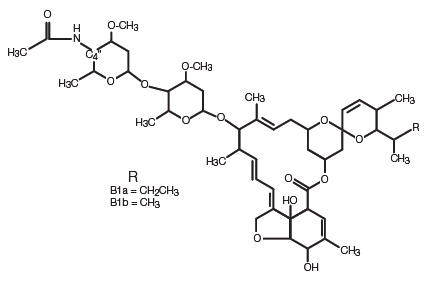
LONGRANGE (eprinomectin) is a ready-to-use, sterile injectable preparation containing eprinomectin, a member of the macrocyclic lactone class of antiparasitics. Each mL of LONGRANGE contains 50 mg of eprinomectin in a co-solvent system of N-methyl-2-pyrrolidone (30% v/v) and triacetin (qs), along with 50 mg of poly-lactide-co-glycolic-acid 75:25 (PLGA), a polymer that allows a slow release of eprinomectin from the formulation, thereby maintaining a prolonged duration of product effectiveness. Butylated hydroxytoluene (0.2 mg/mL) acts as an antioxidant in the formulation.
The chemical name of eprinomectin is 4"-deoxy-4"-epiacetylamino-avermectin B1. It is a semi-synthetic member of the avermectin family of compounds consisting of a mixture of two homologous components, B1a and B1b, which differ by a single methylene group at C26.
-
INDICATIONS FOR USE
LONGRANGE, when administered at the recommended dose volume of 1 mL per 110 lb (50 kg) body weight, is effective in the treatment and control of the following internal and external parasites of cattle:
Gastrointestinal Roundworms Lungworms Bunostomum phlebotomum – Adults and L4
Dictyocaulus viviparus – Adults
Cooperia oncophora – Adults and L4
Cooperia punctata – Adults and L4
Cooperia surnabada – Adults and L4
Grubs
Haemonchus placei – Adults
Hypoderma bovis
Oesophagostomum radiatum – Adults
Ostertagia lyrata – Adults
Mites
Ostertagia ostertagi – Adults, L4, and inhibited L4
Sarcoptes scabiei var. bovis
Trichostrongylus axei – Adults and L4
Trichostrongylus colubriformis – Adults
Persistent Activity
LONGRANGE has been proven to effectively protect cattle from reinfection with the following parasites for the indicated amounts of time following treatment:
Parasites Durations of Persistent Effectiveness Gastrointestinal Roundworms
Bunostomum phlebotomum
150 days
Cooperia oncophora
100 days
Cooperia punctata
100 days
Haemonchus placei
120 days
Oesophagostomum radiatum
120 days
Ostertagia lyrata
120 days
Ostertagia ostertagi
120 days
Trichostrongylus axei
100 days
Lungworms
Dictyocaulus viviparus
150 days
-
DOSAGE AND ADMINISTRATION
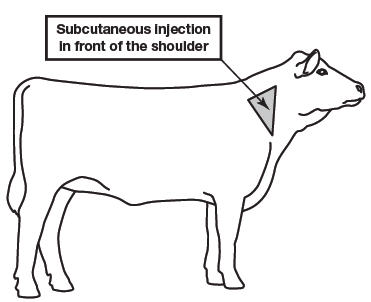
LONGRANGE (eprinomectin) should be given only by subcutaneous injection in front of the shoulder at the recommended dosage level of 1 mg eprinomectin per kg body weight (1 mL per 110 lb body weight).
Each mL of LONGRANGE contains 50 mg of eprinomectin, sufficient to treat 110 lb (50 kg) body weight. Divide doses greater than 10 mL between two injection sites to reduce occasional discomfort or site reaction.
Do not underdose. Ensure each animal receives a complete dose based on a current body weight. Underdosing may result in ineffective treatment, and encourage the development of parasite resistance.
Body Weight (lb) Dose Volume (mL) 110
1
220
2
330
3
440
4
550
5
660
6
770
7
880
8
990
9
1100
10
LONGRANGE is to be given subcutaneously only. Animals should be appropriately restrained to achieve the proper route of administration. Inject under the loose skin in front of the shoulder (see illustration) using a 16 or 18 gauge, ½ to ¾ inch needle.
Sanitize the injection site by applying a suitable disinfectant. Clean, properly disinfected needles should be used to reduce the potential for injection site infections.
100 mL bottle size: Use only polypropylene syringes. Not for use with polycarbonate syringe material. If syringe material is not known, contact the syringe manufacturer prior to use for identification. Alternatively, a 50-mL polypropylene repeater syringe compatible with LONGRANGE may be used. To obtain a list of compatible equipment, contact Boehringer Ingelheim Animal Health USA Inc. at 1-888-637-4251. Do not use beyond 3 months after stopper has been punctured. Discard bottle after 15 stopper punctures. LONGRANGE should not be stored in the repeater syringe.
250 mL and 500 mL bottle sizes: Use only automatic syringe equipment provided by Boehringer Ingelheim Animal Health USA Inc. To obtain compatible equipment, contact Boehringer Ingelheim Animal Health USA Inc. at 1-888-637-4251 or your veterinarian. LONGRANGE should not be stored in automatic syringe equipment. Automatic syringe equipment should be thoroughly cleaned after each use. Discard bottle after one stopper puncture with draw-off spike.
Alternatively, a 50-mL polypropylene repeater syringe compatible with LONGRANGE may be used. To obtain a list of compatible equipment, contact Boehringer Ingelheim Animal Health USA Inc. at 1-888-637-4251. When using a repeater syringe, do not use beyond 3 months after stopper has been punctured. Discard bottle after 15 punctures. LONGRANGE should not be stored in the repeater syringe.
-
WARNINGS AND PRECAUTIONS
Withdrawal Periods and Residue Warnings
Animals intended for human consumption must not be slaughtered within 48 days of the last treatment.
This drug product is not approved for use in female dairy cattle 20 months of age or older, including dry dairy cows. Use in these cattle may cause drug residues in milk and/or in calves born to these cows.
A withdrawal period has not been established for pre-ruminating calves. Do not use in calves to be processed for veal.
User Safety Warnings
Not for Use in Humans. Keep this and all drugs out of the reach of children. Reproductive and developmental toxicities have been reported in laboratory animals following high, repeated exposures to N-methyl-2- pyrrolidone (NMP). Pregnant women should wear gloves and exercise caution or avoid handling this product. The Safety Data Sheet (SDS) contains more detailed occupational safety information.
To report adverse effects, to obtain an SDS, or for assistance, contact Boehringer Ingelheim Animal Health USA Inc. at 1-888-637-4251. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or www.fda.gov/reportanimalae.
Animal Safety Warnings and Precautions
The product is likely to cause tissue damage at the site of injection, including possible granulomas and necrosis. These reactions have disappeared without treatment. Local tissue reaction may result in trim loss of edible tissue at slaughter.
Observe cattle for injection site reactions. If injection site reactions are suspected, consult your veterinarian. This product is not for intravenous or intramuscular use. Protect product from light. LONGRANGE (eprinomectin) has been developed specifically for use in cattle only. This product should not be used in other animal species.
When to Treat Cattle with Grubs
LONGRANGE effectively controls all stages of cattle grubs. However, proper timing of treatment is important. For the most effective results, cattle should be treated as soon as possible after the end of the heel fly (warble fly) season. Destruction of Hypoderma larvae (cattle grubs) at the period when these grubs are in vital areas may cause undesirable host-parasite reactions, including the possibility of fatalities. Killing Hypoderma lineatum when it is in the tissue surrounding the esophagus (gullet) may cause salivation and bloat; killing H. bovis when it is in the vertebral canal may cause staggering or paralysis. These reactions are not specific to treatment with LONGRANGE, but can occur with any successful treatment of grubs. Cattle should be treated either before or after these stages of grub development. Consult your veterinarian concerning the proper time for treatment.
Environmental Hazards
Studies indicate that when eprinomectin comes in contact with soil, it readily and tightly binds to the soil and becomes inactive over time. Free eprinomectin may adversely affect fish and certain aquatic organisms. Do not contaminate water by direct application or by improper disposal of drug containers. Dispose of containers in an approved landfill or by incineration.
As with other avermectins, eprinomectin is excreted in the dung of treated animals and can inhibit the reproduction and growth of pest and beneficial insects that use dung as a source of food and for reproduction. The magnitude and duration of such effects are species and life-cycle specific. When used according to label directions, the product is not expected to have an adverse impact on populations of dung-dependent insects.
Not for use in cattle managed in feedlots or under intensive rotational grazing because the environmental impact has not been evaluated for these scenarios.
Other Warnings: Parasite resistance may develop to any dewormer, and has been reported for most classes of dewormers.
Treatment with a dewormer used in conjunction with parasite management practices appropriate to the geographic area and the animal(s) to be treated may slow the development of parasite resistance.
Fecal examinations or other diagnostic tests and parasite management history should be used to determine if the product is appropriate for the herd, prior to the use of any dewormer. Following the use of any dewormer, effectiveness of treatment should be monitored (for example, with the use of a fecal egg count reduction test or another appropriate method).
A decrease in a drug’s effectiveness over time as calculated by fecal egg count reduction tests may indicate the development of resistance to the dewormer administered. Your parasite management plan should be adjusted accordingly based on regular monitoring.
Macrocyclic lactones provide prolonged drug exposure that may increase selection pressure for resistant parasites. This effect may be more pronounced in extended-release formulations.
-
CLINICAL PHARMACOLOGY
Due to its unique formulation characteristics, when LONGRANGE is injected subcutaneously in the shoulder area of cattle, a polymeric PLGA matrix is formed. The biodegradable matrix solidifies in vivo to form an in situ forming gel, which allows a gradual release of eprinomectin from the formulation. The rate-limiting step is diffusion of the drug through the gel matrix. Because of its mechanism of release, absorption characteristics can be highly dependent upon the injection technique used and the corresponding surface to volume ratio of the gel.
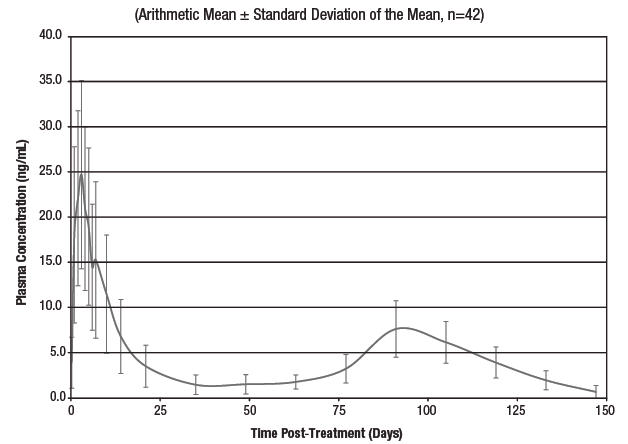
Clinical efficacy of avermectins and milbemycins is closely related to their pharmacokinetic behavior, and the time of parasite exposure to active drug concentrations is relevant to obtain optimal and persistent antiparasitic activity (Lanusse et al., 1997; Lifschitz et al., 1999; Lifschitz et al., 2004; Shoop et al., 1996). Lifschitz et al. (1999) indicated that plasma concentrations between 0.5 and 1 ng/mL would represent the minimal drug level required for optimal nematocidal activity, while others have suggested minimum levels of 1 to 2 ng/mL. Pharmacokinetic studies of LONGRANGE in cattle indicate that effective plasma levels remain for an extended period of time (at least 100 days).
Mean Eprinomectin B1a Plasma Concentration Versus Time Following a Single Subcutaneous Injection of LONGRANGE at a Dose Rate of 1 mg Eprinomectin per kg Body Weight in Beef Cattle
Mode of Action
The macrocyclic lactones have a unique mode of action. Compounds of this class bind selectively and with high affinity to glutamate-gated chloride ion channels that are present in invertebrate nerve and muscle cells. This leads to an increase in the permeability of the cell membrane to chloride ions with hyperpolarization of the nerve or muscle cell, resulting in paralysis and death of the parasite. Compounds of this class may also interact in other ligand-gated chloride ion channels, such as those gated by the neurotransmitter gamma-aminobutyric acid (GABA).
The margin of safety for compounds of this class is at least partially attributable to the fact that mammals do not have glutamate-gated chloride ion channels, and that the macrocyclic lactones have low affinity for other mammalian ligand-gated channels and do not readily cross the blood-brain barrier.
TARGET ANIMAL SAFETY
Clinical studies have demonstrated the wide margin of safety of LONGRANGE (eprinomectin). Overdosing at 3 to 5 times the recommended dose resulted in a statistically significant reduction in average weight gain when compared to the group tested at label dose. Treatment-related lesions observed in most cattle administered the product included swelling, hyperemia, or necrosis in the subcutaneous tissue of the skin. The administration of LONGRANGE at 3 times the recommended therapeutic dose had no adverse reproductive effects on beef cows at all stages of breeding or pregnancy or on their calves.
Not for use in bulls, as reproductive safety testing has not been conducted in males intended for breeding or actively breeding. Not for use in calves less than 3 months of age because safety testing has not been conducted in calves less than 3 months of age.
- HOW SUPPLIED
-
SPL UNCLASSIFIED SECTION
Approved by FDA under NADA # 141-327
Marketed by: Boehringer Ingelheim Animal Health USA Inc., Duluth, GA 30096
The Cattle Head Logo® and LONGRANGE® are registered trademarks of
Boehringer Ingelheim Animal Health USA Inc.
©2022 Boehringer Ingelheim Animal Health USA Inc. All rights reserved.
1050-2889-09, Rev. 07/2022 8LON016F
- Principal Display Panel - 250 mL Display Carton - Front and Side Panel
- Principal Display Panel – 250 mL Display carton – Back and Side Panel
-
INGREDIENTS AND APPEARANCE
LONGRANGE
eprinomectin injection, solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:0010-7216 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength eprinomectin (UNII: 75KP30FD8O) (eprinomectin - UNII:75KP30FD8O) eprinomectin 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength methylpyrrolidone (UNII: JR9CE63FPM) triacetin (UNII: XHX3C3X673) butylated hydroxytoluene (UNII: 1P9D0Z171K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0010-7216-02 1 in 1 CARTON 1 250 mL in 1 BOTTLE, GLASS 2 NDC:0010-7216-03 1 in 1 CARTON 2 500 mL in 1 BOTTLE, GLASS 3 NDC:0010-7216-04 1 in 1 CARTON 3 100 mL in 1 BOTTLE, GLASS Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141327 03/05/2021 Labeler - Boehringer Ingelheim Animal Health USA Inc. (007134091)