Label: HEMORRHOIDAL COCOA BUTTER- hemorrhoidal suppository
- NDC Code(s): 13709-323-01
- Packager: NeilMed Pharmaceuticals Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated August 24, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
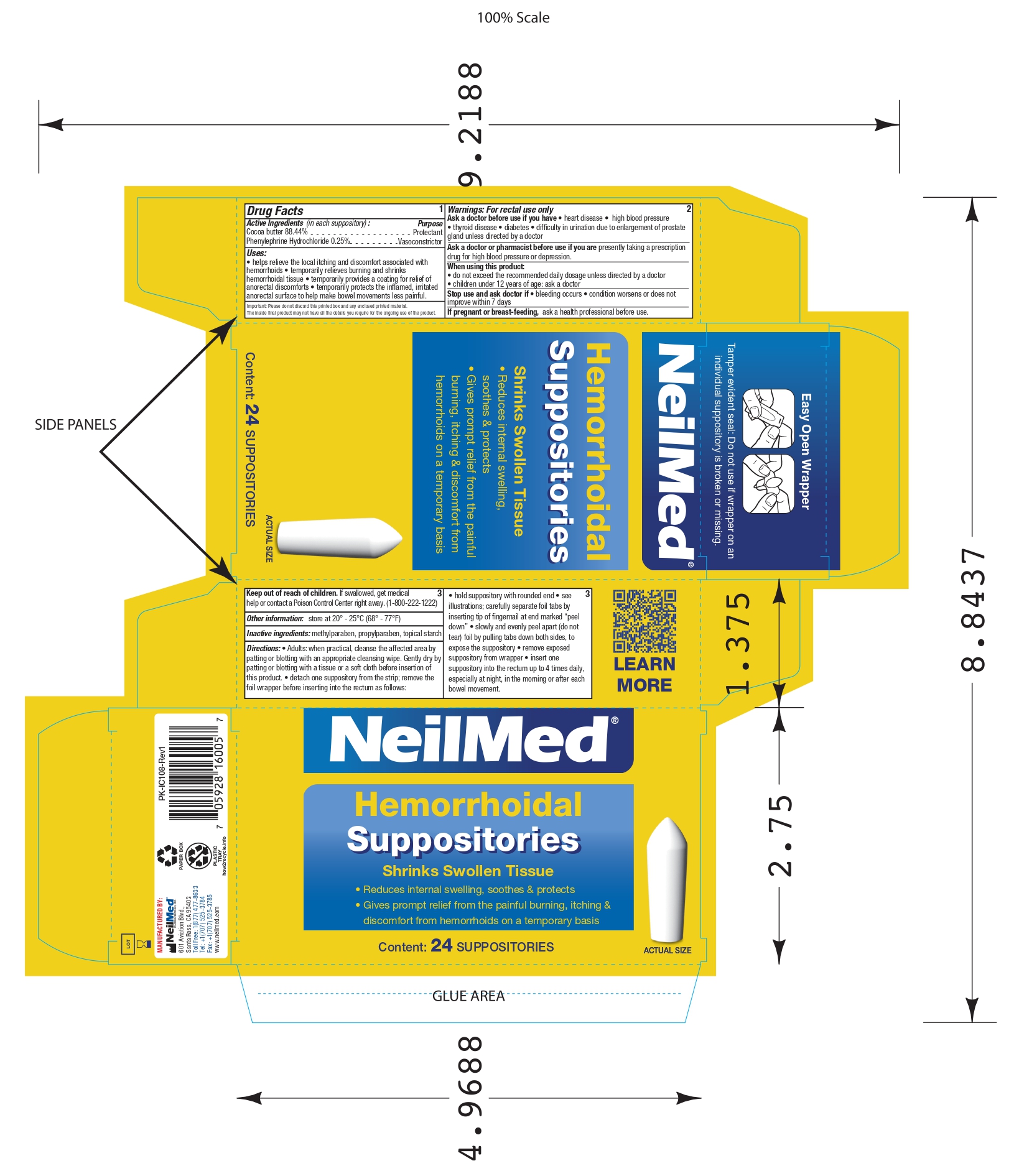
- Drug Facts : Active Ingredients (in each suppository) :
- Purpose
-
Uses:
• helps relieve the local itching and discomfort associated with hemorrhoids • temporarily relieves burning and shrinks hemorrhoidal tissue • temporarily provides a coating for relief of anorectal discomforts • temporarily protects the inflamed, irritatedanorectal surface to help make bowel movements less painful.
- Warnings:
- Ask a doctor before use if you have
- Ask a doctor or pharmacist before use
- When using this product:
- Stop use and ask doctor if
- If pregnant or breast-feeding,
- Keep out of reach of children.
- Other information:
- Inactive ingredients:
-
Directions:
• Adults: when practical, cleanse the affected area by patting or blotting with an appropriate cleansing wipe. Gently dry by patting or blotting with a tissue or a soft cloth before insertion of this product. • detach one suppository from the strip; remove the foil wrapper before inserting into the rectum as follows:
• hold suppository with rounded end • see illustrations; carefully separate foil tabs by inserting tip of fingernail at end marked “peel
down” • slowly and evenly peel apart (do not tear) foil by pulling tabs down both sides, to expose the suppository • remove exposed
suppository from wrapper • insert one suppository into the rectum up to 4 times daily, especially at night, in the morning or after each
bowel movement. - INTENDED USE OF THE DEVICE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HEMORRHOIDAL COCOA BUTTER
hemorrhoidal suppositoryProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13709-323 Route of Administration RECTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 2.5 mg in 1 g COCOA BUTTER (UNII: 512OYT1CRR) (COCOA BUTTER - UNII:512OYT1CRR) COCOA BUTTER 884.4 mg in 1 g Inactive Ingredients Ingredient Name Strength METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) MODIFIED CORN STARCH (1-OCTENYL SUCCINIC ANHYDRIDE) (UNII: 461P5CJN6T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13709-323-01 4 in 1 CARTON 08/24/2023 1 6 in 1 BLISTER PACK 1 2 g in 1 CAPSULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M015 08/24/2023 Labeler - NeilMed Pharmaceuticals Inc. (799295915) Establishment Name Address ID/FEI Business Operations NeilMed Pharmaceuticals Inc. 799295915 manufacture(13709-323)