Label: RE/COVER KIT- colloidal oatmeal kit
- NDC Code(s): 10596-901-03
- Packager: KAO USA
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated August 22, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other information
-
Inactive ingredients
water, glycerin, cetearyl alcohol, petrolatum, stearic acid, C12-15 alkyl benzoate, dimethicone, aesculus hippocastanum (horse chestnut) seed extract, laureth-3, hydroxyacetophenone, ceteareth-20, allantoin, arginine, sodium hydroxide, carbomer, panthenol, tetrasodium glutamate diacetate, phenoxyethanol
- Questions or comments?
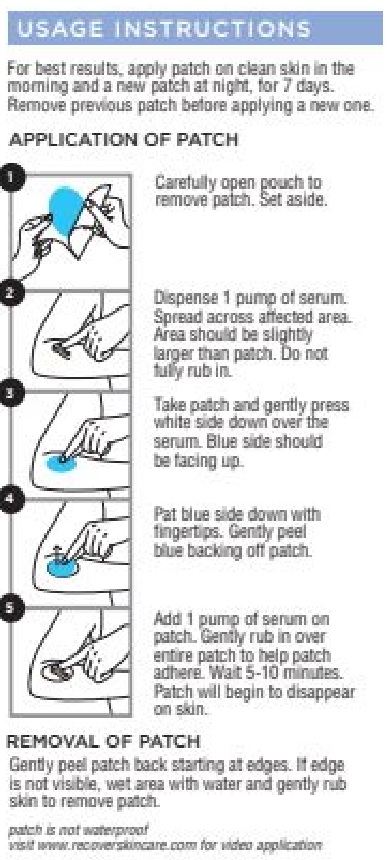
- Moisture Patch Instructions
- MOISTURE PATCH INGREDIENTS
-
PRINCIPAL DISPLAY PANEL
re/cover™
ECZEMA RELIEF SYSTEM
Fast-acting system quickly relieves the disruption and discomfort of eczema with a soothing serum and invisible protective moisture patch to help recover healthy-looking skin
+CALMING ECZEMA SERUM
COLLOIDAL OATMEAL SKIN PROTECTANT
+MOISTURE PATCHES
- Serum helps to shorten eczema flare ups and relieve the itch
- Dermatologist Tested
- Steroid Free
7 DAY SYSTEM
14 MOISTURE PATCHES
0.30 FL OZ (9 mL) CALMING ECZEMA SERUM
For additional information:
www.recoverskincare.com
or 1-800-309-1638
RE/COVER is a trademark of Kao Corp.
Distributed by Kao USA Inc.
Cincinnati, OH 45214
©2023
Patch: Made in Japan.
Serum: Made in USA of US & Imported Ingredients

-
INGREDIENTS AND APPEARANCE
RE/COVER KIT
colloidal oatmeal kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10596-901 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10596-901-03 1 in 1 KIT; Type 0: Not a Combination Product 08/21/2023 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 0 BOTTLE, WITH APPLICATOR 1 mL Part 1 of 1 RE/COVER SERUM
colloidal oatmeal emulsionProduct Information Item Code (Source) NDC:10596-902 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OATMEAL (UNII: 8PI54V663Y) (OATMEAL - UNII:8PI54V663Y) OATMEAL 1 mg in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) PETROLATUM (UNII: 4T6H12BN9U) DIMETHICONE (UNII: 92RU3N3Y1O) HORSE CHESTNUT (UNII: 3C18L6RJAZ) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) ARGININE (UNII: 94ZLA3W45F) SODIUM HYDROXIDE (UNII: 55X04QC32I) PHENOXYETHANOL (UNII: HIE492ZZ3T) LAURETH-3 (UNII: F32E4CB0UJ) STEARIC ACID (UNII: 4ELV7Z65AP) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) ALLANTOIN (UNII: 344S277G0Z) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) PANTHENOL (UNII: WV9CM0O67Z) TETRASODIUM GLUTAMATE DIACETATE (UNII: 5EHL50I4MY) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 9 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M016 08/21/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 08/21/2023 Labeler - KAO USA (004251617)