Label: DOVE- raw shea butter and hyaluronic acid moisturizing 48h antiperspirant deodorant stick
- NDC Code(s): 64942-2147-1
- Packager: Conopco Inc. d/b/a/ Unilever
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 21, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
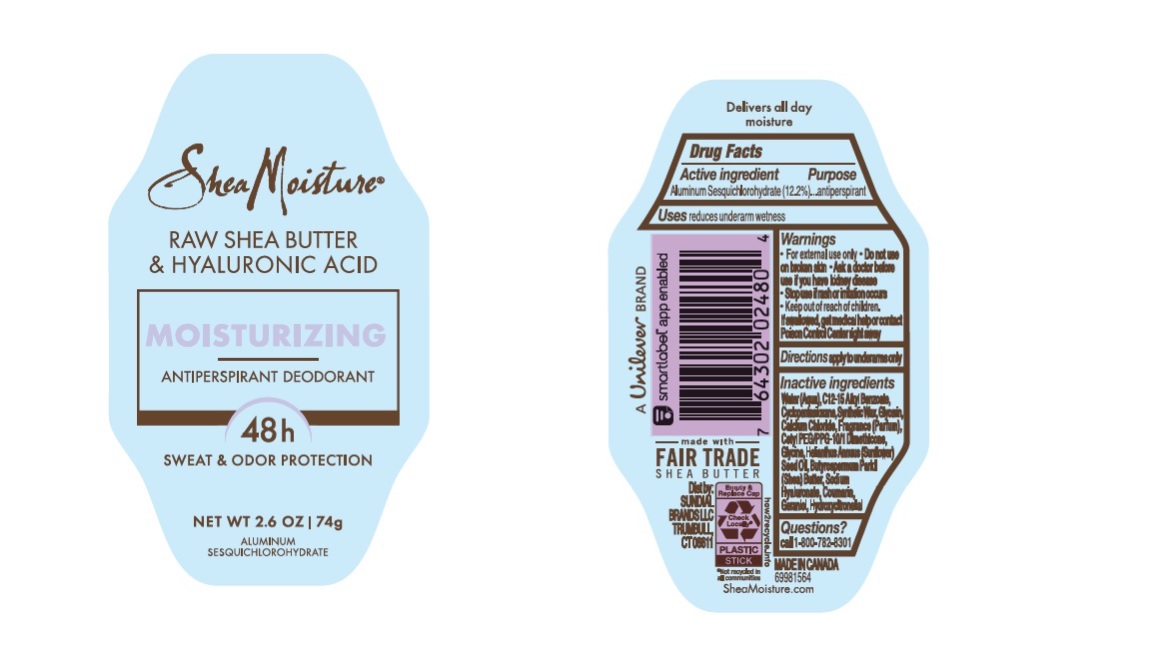
- SHEA MOISTURE RAW SHEA BUTTER & HYALURONIC ACID MOISTURIZING 48H ANTIPERSPIRANT DEODORANT - aluminum sesquichlorohydrate stick
- Drug Facts
- Purpose
- Uses
- Warnings
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Inactive ingredients
- Questions?
- Packaging
-
INGREDIENTS AND APPEARANCE
DOVE
raw shea butter and hyaluronic acid moisturizing 48h antiperspirant deodorant stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:64942-2147 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM SESQUICHLOROHYDRATE (UNII: UCN889409V) (ALUMINUM SESQUICHLOROHYDRATE - UNII:UCN889409V) ALUMINUM SESQUICHLOROHYDRATE 12.2 g in 100 g Inactive Ingredients Ingredient Name Strength CALCIUM CHLORIDE (UNII: M4I0D6VV5M) WATER (UNII: 059QF0KO0R) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) SYNTHETIC WAX (1200 MW) (UNII: Q3Z4BCH099) GLYCERIN (UNII: PDC6A3C0OX) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 4) (UNII: 8INO2K35FA) GLYCINE (UNII: TE7660XO1C) SUNFLOWER OIL (UNII: 3W1JG795YI) SHEA BUTTER (UNII: K49155WL9Y) HYALURONATE SODIUM (UNII: YSE9PPT4TH) GERANIOL (UNII: L837108USY) HYDROXYCITRONELLAL (UNII: 8SQ0VA4YUR) COUMARIN (UNII: A4VZ22K1WT) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64942-2147-1 74 g in 1 CONTAINER; Type 0: Not a Combination Product 10/01/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M019 10/01/2023 Labeler - Conopco Inc. d/b/a/ Unilever (001375088) Registrant - Conopco Inc. d/b/a/ Unilever (001375088) Establishment Name Address ID/FEI Business Operations KDC/ONE Development Corporation, Inc 204006464 manufacture(64942-2147)