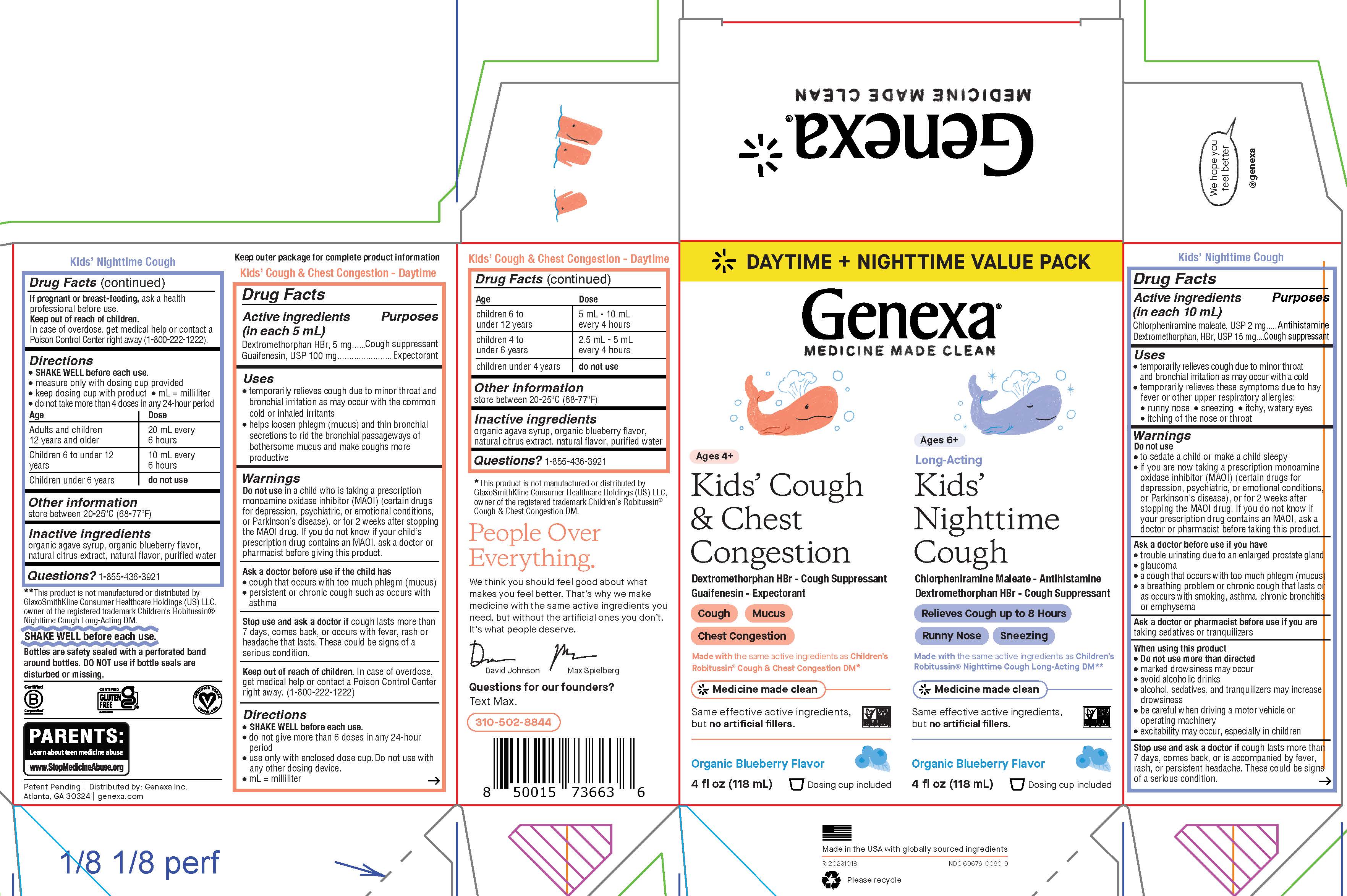
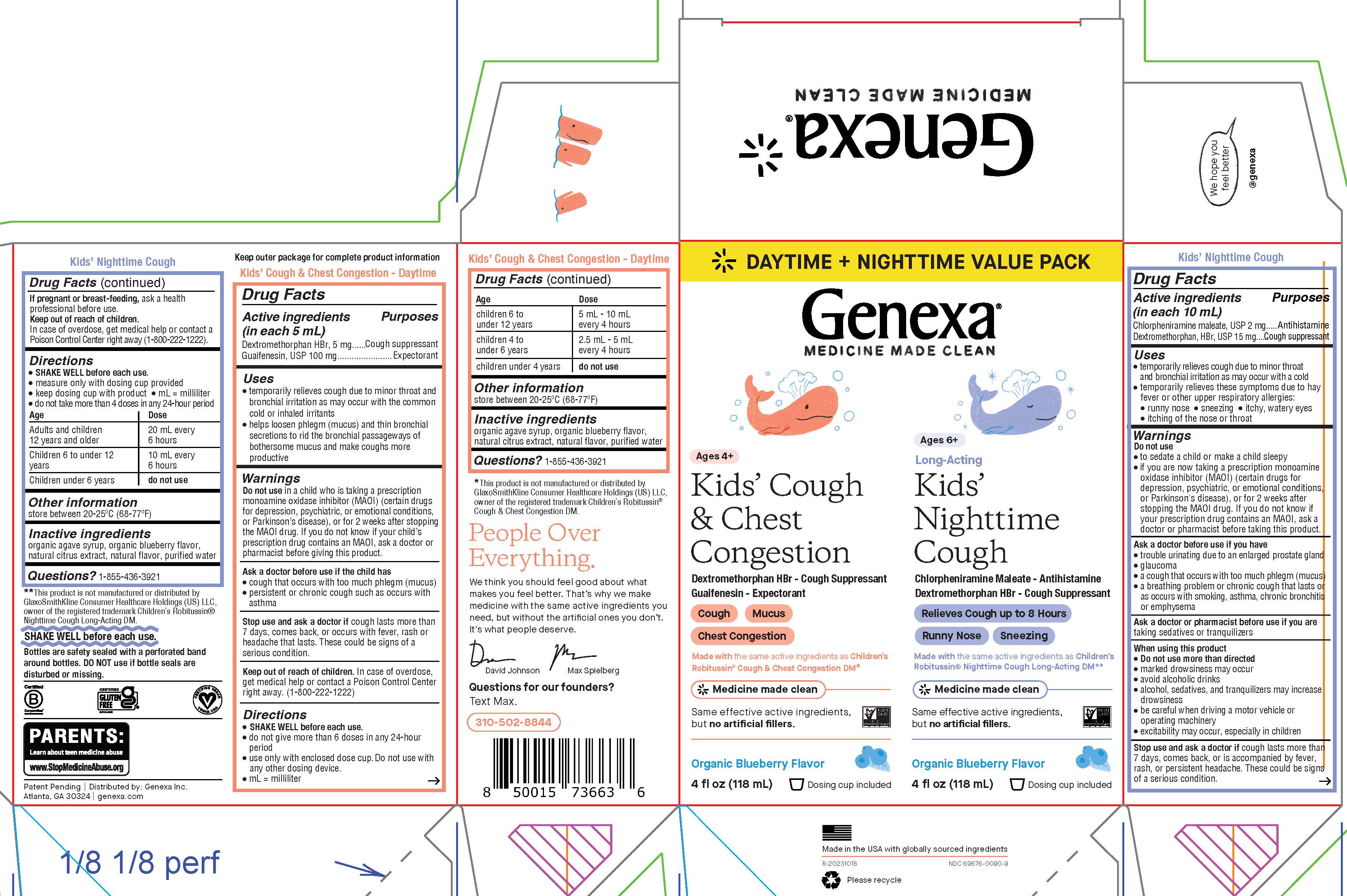
Label: KIDS COUGH DAYTIME NIGHTTIME COMBO- dextromethorphan hbr, guaifenesin, chlorpheniramine maleate kit
- NDC Code(s): 69676-0090-9
- Packager: Genexa Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
Do not use in a child who is taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your child’s prescription drug contains an MAOI, ask a doctor or pharmacist before giving this product.
Ask a doctor before use if the child has
- cough that occurs with too much phlegm (mucus)
- persistent or chronic cough such as occurs with asthma
-
DOSAGE & ADMINISTRATION
Directions
- SHAKE WELL before each use
- do not give more than 6 doses in any 24-hour period
- use only with enclosed dose cup. Do not use with any other dosing device.
- mL = milliliter
Age Dose children 6 to under 12 years 5 mL - 10 mL every 4 hours children 4 to under 6 years 2.5 mL - 5 mL every 4 hours children under 4 years do not use - STORAGE AND HANDLING
- INACTIVE INGREDIENT
- QUESTIONS
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
Do not use
• to sedate a child or make a child sleepy
• if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
• trouble urinating due to an enlarged prostate gland
• glaucoma
• a cough that occurs with too much phlegm (mucus)
• a breathing problem or chronic cough that lasts or as occurs with smoking, asthma, chronic bronchitis or emphysema
When using this product
• Do not use more than directed
• marked drowsiness may occur
• avoid alcoholic drinks
• alcohol, sedatives, and tranquilizers may increase drowsiness
• be careful when driving a motor vehicle or operating machinery
• excitability may occur, especially in children
-
DOSAGE & ADMINISTRATION
Directions
- SHAKE WELL before each use.
- measure only with dosing cup provided
- keep dosing cup with product
- mL = milliliter
- do not take more than 4 doses in any 24-hour period
Age Dose Adults and children
12 years and older
20 mL every
6 hours
Children 6 to under 12
years
10 mL every 6 hours Children under 6 years do not use - STORAGE AND HANDLING
- INACTIVE INGREDIENT
- QUESTIONS
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL
DAYTIME + NIGHTTIME VALUE PACK
Genexa®
MEDICINE MADE CLEAN
Ages 4+
Kids' Cough & Chest CongestionDextromethorphan HBr - Cough Suppressant
Guaifenesin - Expectorant
Cough
Mucus
Chest Congestion
Made with the same active ingredients as Children's Robitussin® Cough & Chest Congestion DM*
Medicine made clean
Same effective active ingredients, but no artificial fillers.
Organic Blueberry Flavor
4 fl oz (118 mL)
Dosing cup included
Ages 6+
Long-Acting
Kids' Nighttime CoughChlorpheniramine Maleate - Antihistamine
Dextromethorphan HBr - Cough Suppressant
Relieves Cough up to 8 Hours
Runny Nose
Sneezing
Made with the same active ingredients as Children's Robitussin® Nighttime Cough Long-Acting DM**
Medicine made clean
Same effective active ingredients, but no artificial fillers.
Organic Blueberry Flavor
4 fl oz (118 mL)
Dosing cup included
-
INGREDIENTS AND APPEARANCE
KIDS COUGH DAYTIME NIGHTTIME COMBO
dextromethorphan hbr, guaifenesin, chlorpheniramine maleate kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69676-0090 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69676-0090-9 1 in 1 KIT 01/26/2024 1 1 in 1 BOTTLE; Type 0: Not a Combination Product Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 0 BOTTLE, PLASTIC 1 mL in 5 Part 2 0 BOTTLE 1 mL in 10 Part 1 of 2 KIDS COUGH AND CHEST CONGESTION
dextromethorphan hbr, guaifenesin liquidProduct Information Item Code (Source) NDC:69676-0037 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 5 mg in 5 mL GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 100 mg in 5 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CITRUS FRUIT (UNII: XDK00Z8012) AGAVE TEQUILANA STEM (UNII: J026JA743Y) Product Characteristics Color brown (GOLDEN) Score Shape Size Flavor BLUEBERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 118 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M013 01/26/2024 Part 2 of 2 NIGHTTIME COUGH-KIDS
chlorpheniramine maleate, dextromethorphan hbr solutionProduct Information Item Code (Source) NDC:69676-0088 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORPHENIRAMINE MALEATE (UNII: V1Q0O9OJ9Z) (CHLORPHENIRAMINE - UNII:3U6IO1965U) CHLORPHENIRAMINE MALEATE 2 mg in 10 mL DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 15 mg in 10 mL Inactive Ingredients Ingredient Name Strength CITRUS FRUIT (UNII: XDK00Z8012) WATER (UNII: 059QF0KO0R) AGAVE TEQUILANA STEM (UNII: J026JA743Y) Product Characteristics Color brown (GOLDEN) Score Shape Size Flavor BLUEBERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 118 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M013 01/26/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M013 01/26/2024 Labeler - Genexa Inc. (079751024)