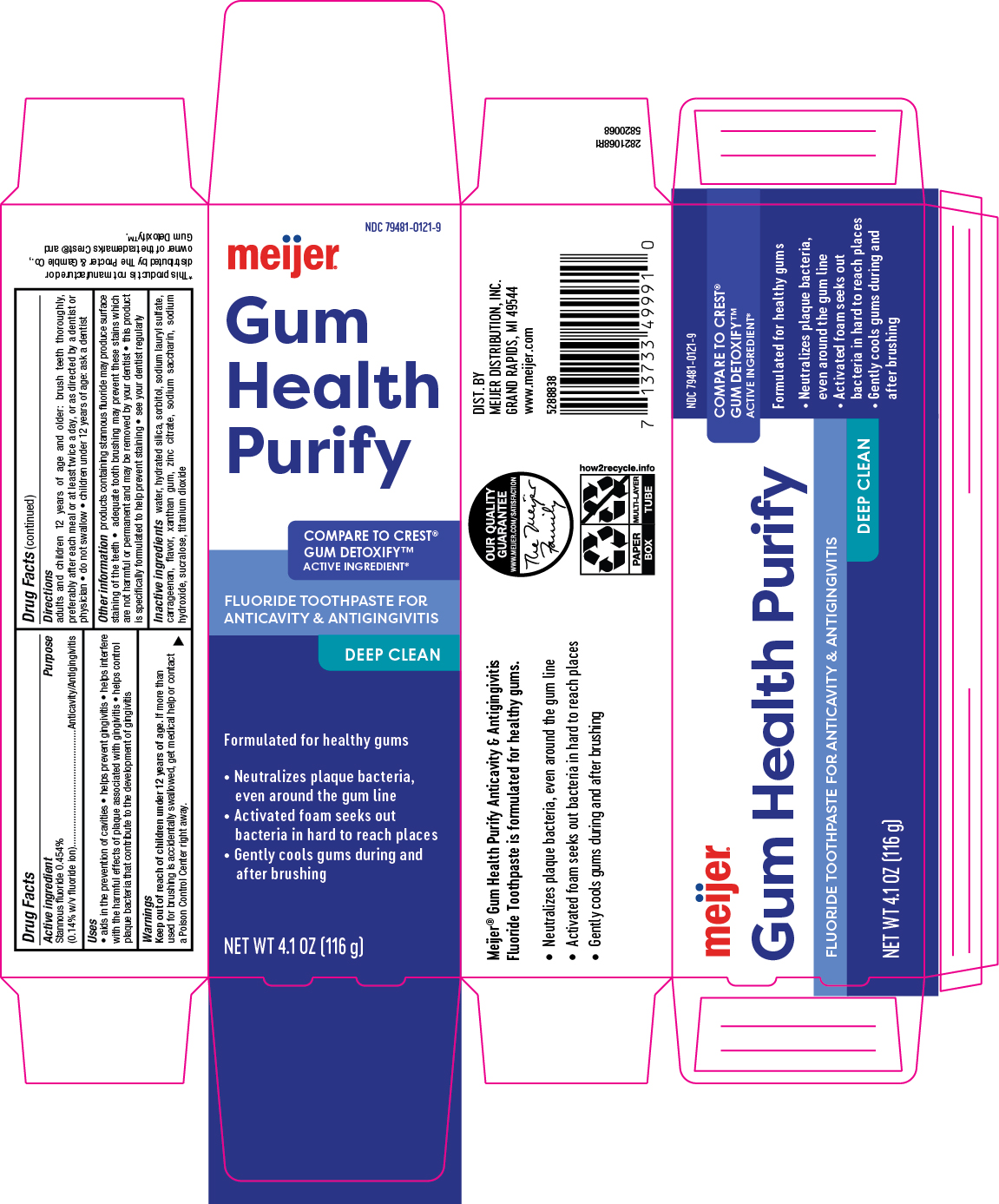
Label: GUM DETOXIFY- stannous fluoride gel, dentifrice
- NDC Code(s): 79481-0121-9
- Packager: Meijer, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 30, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
- Keep out of reach of children under 12 years of age
- Directions
- Other information
- Inactive ingredients
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
GUM DETOXIFY
stannous fluoride gel, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:79481-0121 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength STANNOUS FLUORIDE (UNII: 3FTR44B32Q) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.14 g in 100 g Inactive Ingredients Ingredient Name Strength SACCHARIN SODIUM (UNII: SB8ZUX40TY) SODIUM HYDROXIDE (UNII: 55X04QC32I) XANTHAN GUM (UNII: TTV12P4NEE) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) HYDRATED SILICA (UNII: Y6O7T4G8P9) ZINC CITRATE (UNII: K72I3DEX9B) SODIUM LAURYL SULFATE (UNII: 368GB5141J) WATER (UNII: 059QF0KO0R) CARRAGEENAN (UNII: 5C69YCD2YJ) Product Characteristics Color white (Off-white) Score Shape Size Flavor MINT (Sweet mint) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:79481-0121-9 1 in 1 CARTON 02/16/2024 1 116 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M021 02/16/2024 Labeler - Meijer, Inc. (006959555) Registrant - Lornamead (080046418) Establishment Name Address ID/FEI Business Operations Lornamead 080046418 pack(79481-0121) , manufacture(79481-0121)