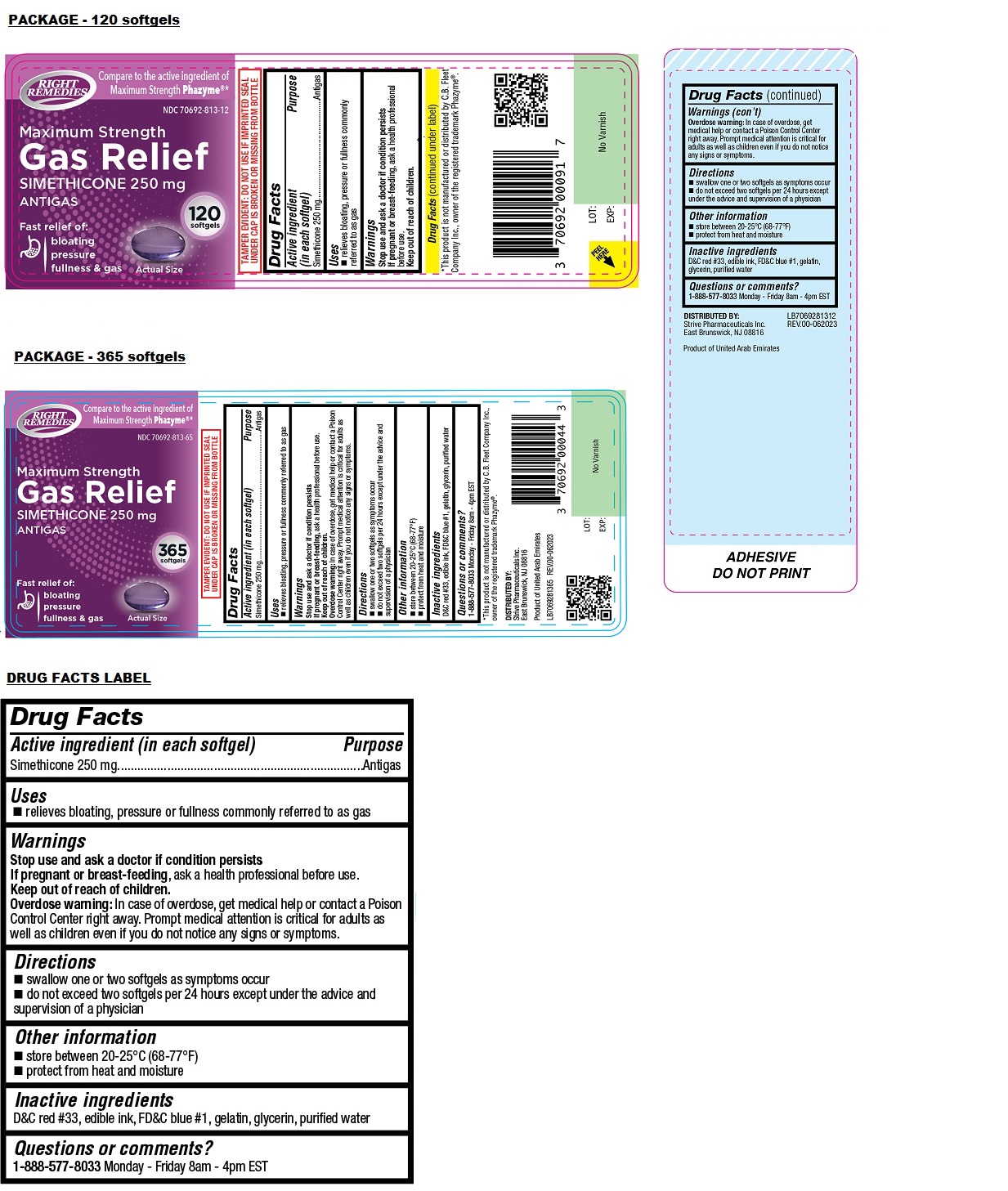
Label: RIGHT REMEDIES MAXIMUM STRENGTH GAS RELIEF SOFTGEL- simethicone capsule, liquid filled
- NDC Code(s): 70692-813-12, 70692-813-65
- Packager: Strive Pharmaceuticals Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
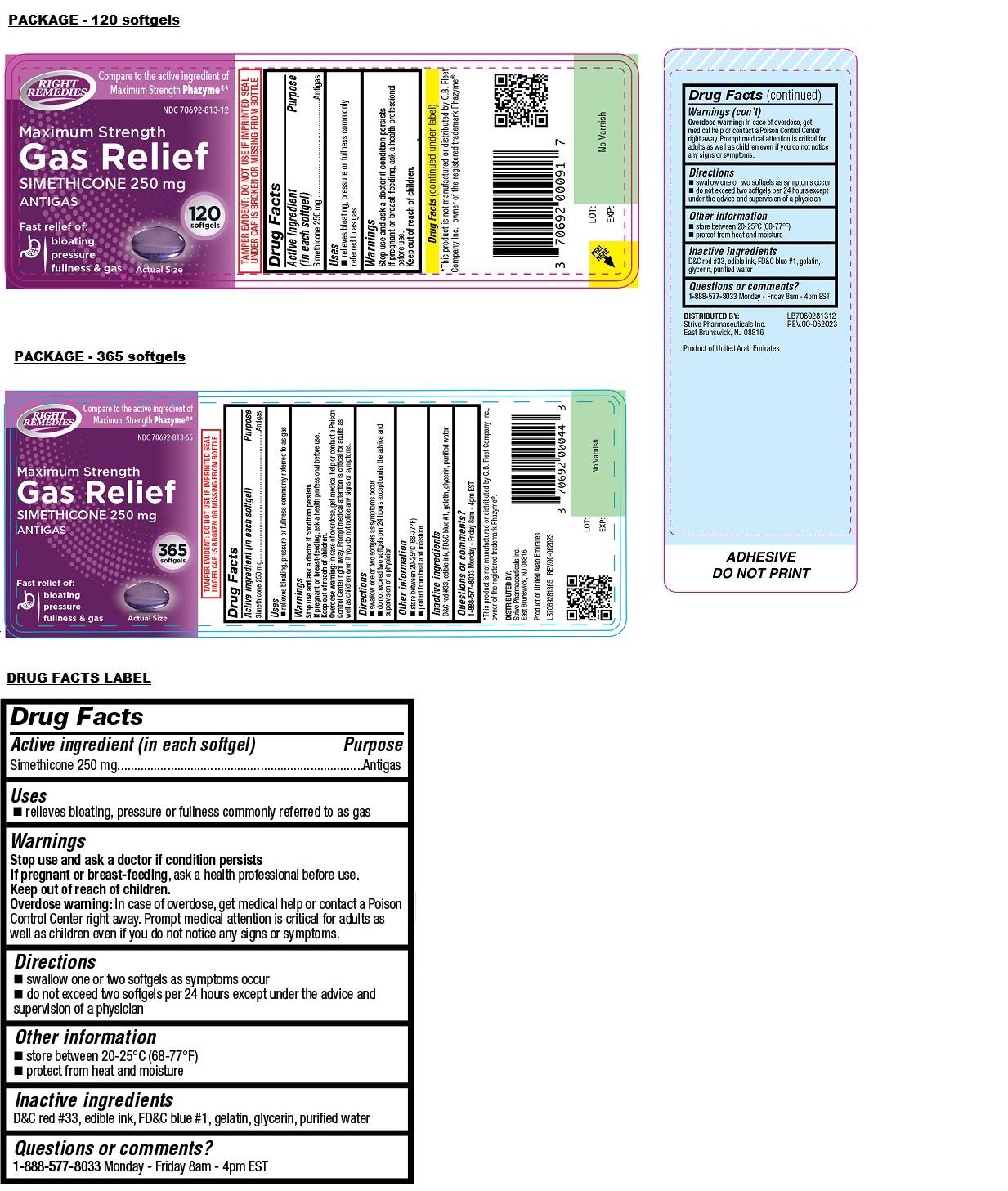
- Drug Facts
- Active ingredient (in each softgel)
- Purpose
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
SPL UNCLASSIFIED SECTION
Compare to the active ingredient of Maximum Strength Phazyme®*
Fast relief of:
bloating
pressure
fullness & gasTAMPER EVIDENT: DO NOT USE IF IMPRINTED SEAL UNDER CAP IS BROKEN OR MISSING FROM BOTTLE
*This product is not manufactured or distributed by C.B. Fleet Company Inc., owner of the registered trademark Phazyme®.
DISTRIBUTED BY:
Strive Pharmaceuticals Inc.
East Brunswick, NJ 08816Product of United Arab Emirates
- Packaging
-
INGREDIENTS AND APPEARANCE
RIGHT REMEDIES MAXIMUM STRENGTH GAS RELIEF SOFTGEL
simethicone capsule, liquid filledProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70692-813 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 250 mg Inactive Ingredients Ingredient Name Strength D&C RED NO. 33 (UNII: 9DBA0SBB0L) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) Product Characteristics Color purple Score no score Shape OVAL Size 12mm Flavor Imprint Code 813 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70692-813-12 120 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 02/05/2024 2 NDC:70692-813-65 365 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 02/05/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M002 02/05/2024 Labeler - Strive Pharmaceuticals Inc. (080028013)