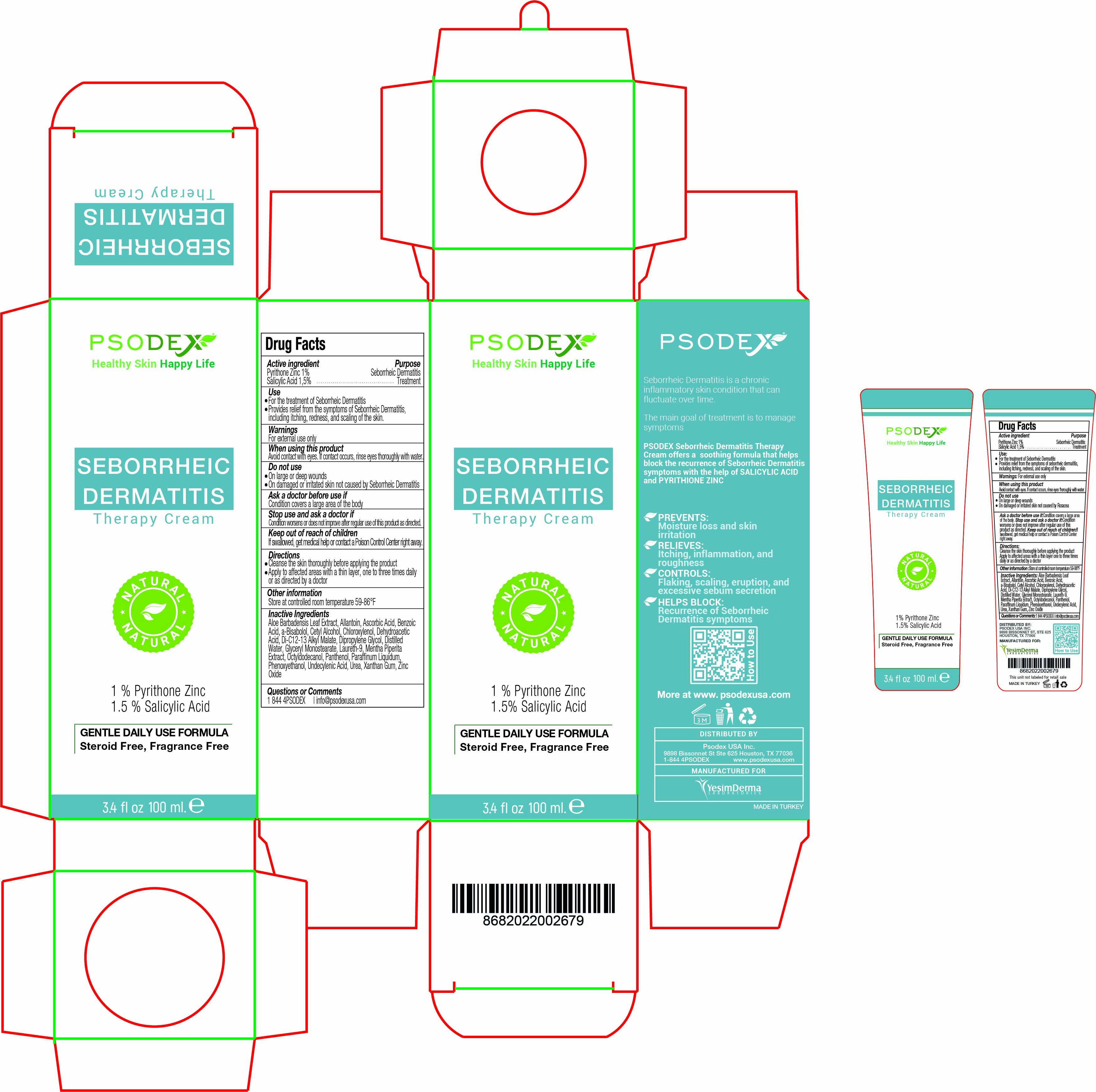
Label: SEBORRHEIC DERMATITIS THERAPY- salicylic acid, pyrithione zinc cream
- NDC Code(s): 73503-010-00, 73503-010-01
- Packager: PSODEX USA INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Inactive Ingredients
Aloe Barbadensis Leaf Extract, Allantoin, Ascorbic Acid, Benzoic
Acid, a-Bisabolol, Cetyl Alcohol, Chloroxylenol, Dehydroacetic
Acid, Di-C12-13 Alkyl Malate, Dipropylene Glycol, Distilled
Water, Glyceryl Monostearate, Laureth-9, Mentha Piperita
Extract, Octyldodecanol, Panthenol, Paraffinum Liquidum,
Phenoxyethanol, Undecylenic Acid, Urea, Xanthan Gum, Zinc
Oxide -
DESCRIPTION
Seborrheic Dermatitis is a chronic inflammatory skin condition that can fluctuate over time.
The main goal of treatment is to manage symptoms
PSODEX Seborrheic Dermatitis Therapy Cream offers a soothing formula that helps block the recurrence of Seborrheic Dermatitis symptoms with the help of SALICYLIC ACID and PYRITHIONE ZINC
PREVENTS:
Moisture loss and skin irritation
RELIEVES:
Itching, inflammation, and roughness
CONTROLS:
Flaking, scaling, eruption, and excessive sebum secretion
HELPS BLOCK:
Recurrence of Seborrheic Dermatitis symptoms - INFORMATION FOR OWNERS/CAREGIVERS
- Active ingredient
- Active ingredient
- Use
- Directions
- Ask a doctor before use if
- Stop use and ask a doctor if
- Keep out of reach of children
- Do not use
- When using this product
- Other information
- Questions or Comments
- Warnings
- Purpose
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SEBORRHEIC DERMATITIS THERAPY
salicylic acid, pyrithione zinc creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73503-010 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PYRITHIONE ZINC (UNII: R953O2RHZ5) (PYRITHIONE ZINC - UNII:R953O2RHZ5) PYRITHIONE ZINC 1 g in 100 mL SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 1.5 g in 100 mL Inactive Ingredients Ingredient Name Strength DIPROPYLENE GLYCOL (UNII: E107L85C40) 0.2 g in 100 mL UNDECYLENIC ACID (UNII: K3D86KJ24N) 2 g in 100 mL ZINC OXIDE (UNII: SOI2LOH54Z) 9 g in 100 mL ALLANTOIN (UNII: 344S277G0Z) 2 g in 100 mL MENTHA PIPERITA LEAF (UNII: A389O33LX6) 5 g in 100 mL XANTHAN GUM (UNII: TTV12P4NEE) 0.3 g in 100 mL CETYL ALCOHOL (UNII: 936JST6JCN) 5 g in 100 mL DI-C12-13 ALKYL MALATE (UNII: RRL7C51WPD) 2 g in 100 mL OCTYLDODECANOL (UNII: 461N1O614Y) 2 g in 100 mL ASCORBIC ACID (UNII: PQ6CK8PD0R) 0.5 g in 100 mL ALOE VERA LEAF (UNII: ZY81Z83H0X) 5 g in 100 mL PANTHENOL (UNII: WV9CM0O67Z) 1 g in 100 mL MINERAL OIL (UNII: T5L8T28FGP) 5 g in 100 mL PHENOXYETHANOL (UNII: HIE492ZZ3T) 0.5 g in 100 mL UREA (UNII: 8W8T17847W) 5 g in 100 mL LEVOMENOL (UNII: 24WE03BX2T) 2 g in 100 mL GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) 2 g in 100 mL CHLOROXYLENOL (UNII: 0F32U78V2Q) 0.5 g in 100 mL BENZOIC ACID (UNII: 8SKN0B0MIM) 0.25 g in 100 mL DEHYDROACETIC ACID (UNII: 2KAG279R6R) 0.25 g in 100 mL WATER (UNII: 059QF0KO0R) 45 mL in 100 mL POLIDOCANOL (UNII: 0AWH8BFG9A) 3 g in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73503-010-01 1000 in 1 BOX 01/01/2024 1 NDC:73503-010-00 1000 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M032 01/01/2024 Labeler - PSODEX USA INC (076051073) Registrant - PSODEX USA INC (076051073) Establishment Name Address ID/FEI Business Operations BERKO ILAC VE KIMYA SANAYI ANONIM SIRKETI-SULTANBEYLI SUBESI 533135007 manufacture(73503-010)