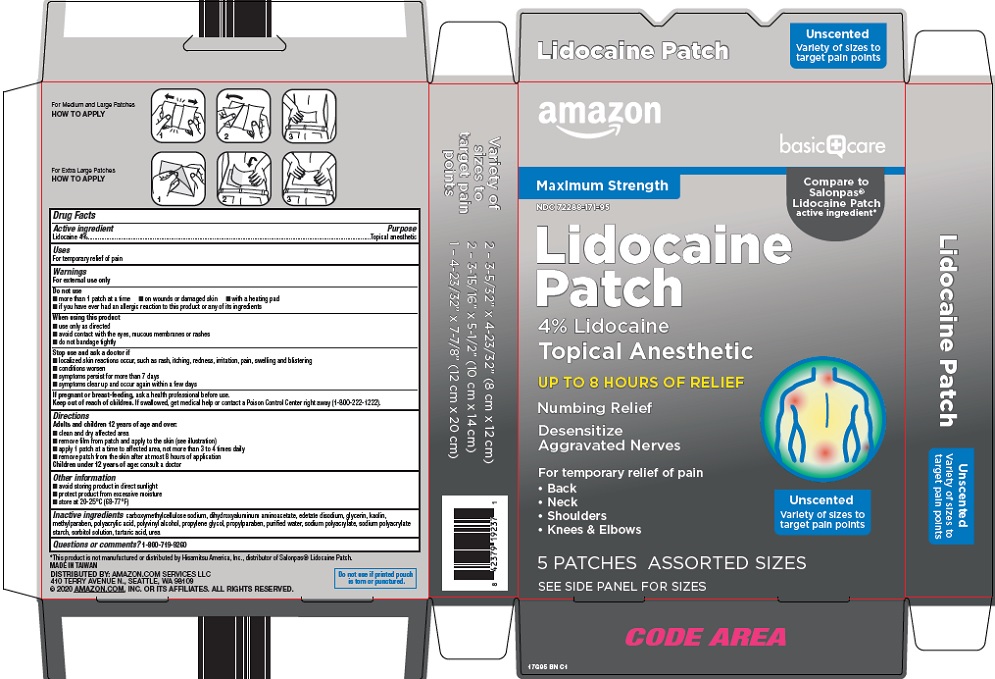
Label: BASIC CARE LIDOCAINE- lidocaine kit
- NDC Code(s): 72288-171-95, 72288-250-00, 72288-413-00, 72288-750-00
- Packager: Amazon.com Services LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 17, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only
Do not use
- •
- more than 1 patch at a time
- •
- on wounds or damaged skin
- •
- with a heating pad
- •
- if you have ever had an allergic reaction to this product or any of its ingredients
When using this product
- •
- use only as directed
- •
- avoid contact with the eyes, mucous membranes or rashes
- •
- do not bandage tightly
-
Directions
Adults and children 12 years of age and over:
- •
- clean and dry affected area
- •
- remove film from patch and apply to the skin (see illustration)
- •
- apply 1 patch at a time to affected area, not more than 3 to 4 times daily
- •
- remove patch from the skin after at most 8 hours of application
Children under 12 years of age: consult a doctor
- Other information
- Inactive ingredients
- Questions or comments?
-
Package/Label Principal Display Panel
Maximum Strength
Compare to Salonpas® Lidocaine Patch active ingredient
Lidocaine Patch
4% Lidocaine
Topical Anesthetic
UP TO 8 HOURS OF RELIEF
Numbing Relief
Desensitize Aggravated Nerves
For temporary relief of pain
- Back
- Neck
- Shoulders
- Knees & Elbows
Unscented
Variety of sizes to target pain points
5 PATCHES ASSORTED SIZES
SEE SIDE PANEL FOR SIZES
-
INGREDIENTS AND APPEARANCE
BASIC CARE LIDOCAINE
lidocaine kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72288-171 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72288-171-95 1 in 1 CARTON; Type 0: Not a Combination Product 03/18/2021 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 2 POUCH 2 Part 2 2 POUCH 2 Part 3 1 POUCH 1 Part 1 of 3 BASIC CARE LIDOCAINE
lidocaine patchProduct Information Item Code (Source) NDC:72288-250 Route of Administration TOPICAL, PERCUTANEOUS, TRANSDERMAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 384 mg Inactive Ingredients Ingredient Name Strength CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) DIHYDROXYALUMINUM AMINOACETATE (UNII: DO250MG0W6) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) KAOLIN (UNII: 24H4NWX5CO) METHYLPARABEN (UNII: A2I8C7HI9T) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) TARTARIC ACID (UNII: W4888I119H) UREA (UNII: 8W8T17847W) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72288-250-00 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 Part 2 of 3 BASIC CARE LIDOCAINE
lidocaine patchProduct Information Item Code (Source) NDC:72288-750 Route of Administration TOPICAL, PERCUTANEOUS, TRANSDERMAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 560 mg Inactive Ingredients Ingredient Name Strength CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) DIHYDROXYALUMINUM AMINOACETATE (UNII: DO250MG0W6) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) KAOLIN (UNII: 24H4NWX5CO) METHYLPARABEN (UNII: A2I8C7HI9T) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) TARTARIC ACID (UNII: W4888I119H) UREA (UNII: 8W8T17847W) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72288-750-00 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 Part 3 of 3 BASIC CARE LIDOCAINE
lidocaine patchProduct Information Item Code (Source) NDC:72288-413 Route of Administration TOPICAL, PERCUTANEOUS, TRANSDERMAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 960 mg Inactive Ingredients Ingredient Name Strength CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) DIHYDROXYALUMINUM AMINOACETATE (UNII: DO250MG0W6) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) KAOLIN (UNII: 24H4NWX5CO) METHYLPARABEN (UNII: A2I8C7HI9T) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) TARTARIC ACID (UNII: W4888I119H) UREA (UNII: 8W8T17847W) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72288-413-00 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 03/18/2021 Labeler - Amazon.com Services LLC (128990418)