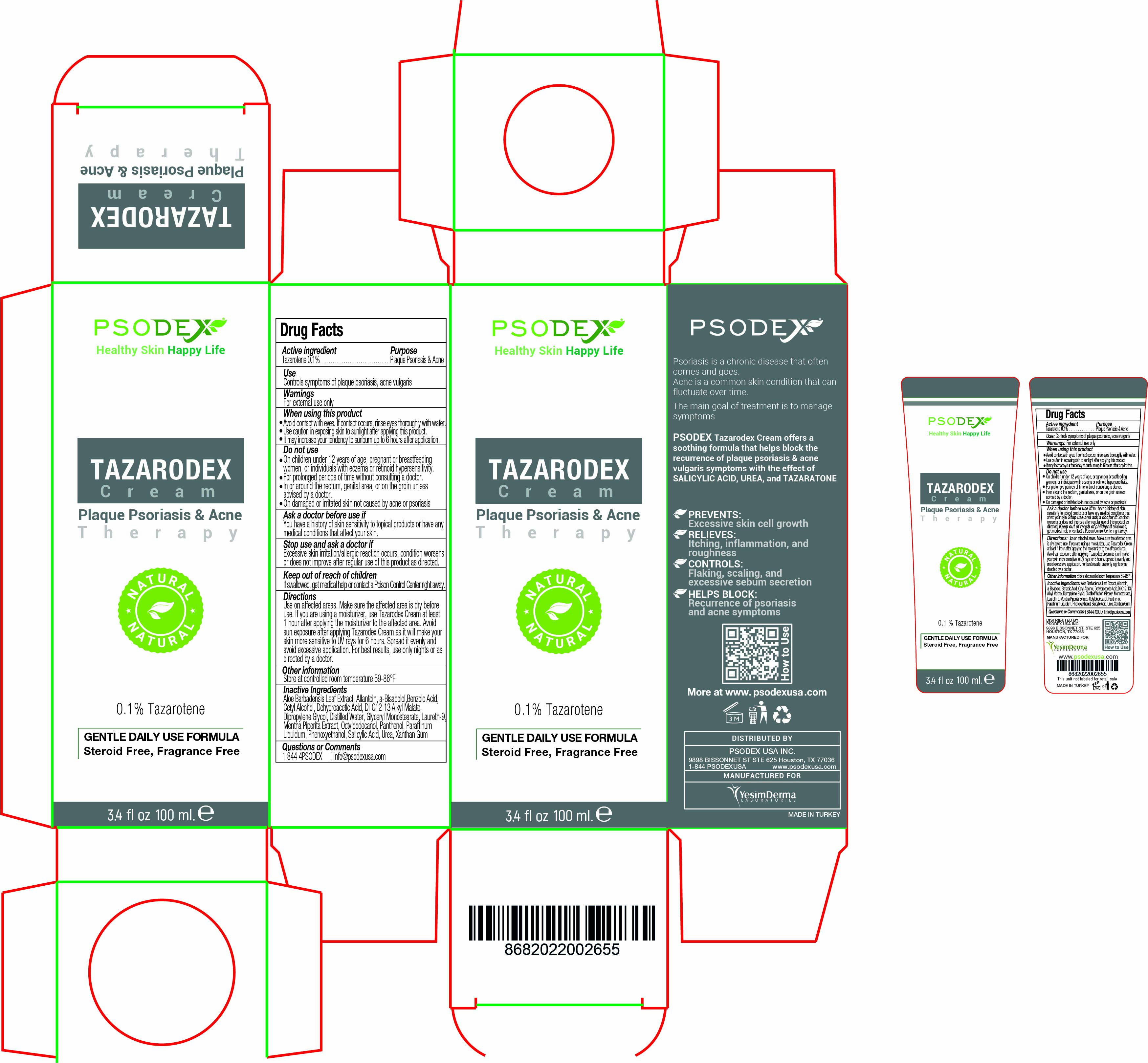
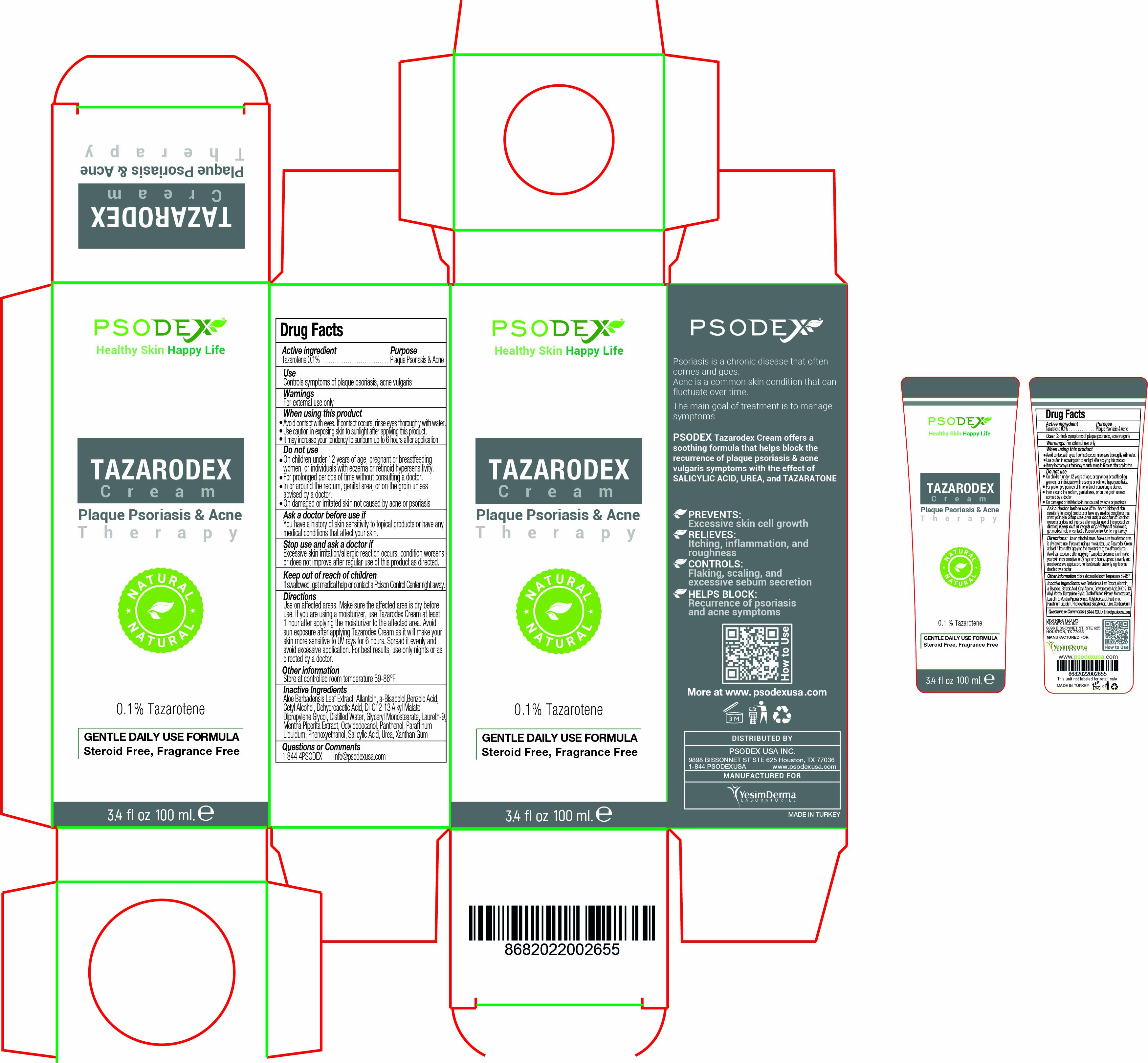
Label: TAZARODEX PLAQUE PSORIASIS AND ACNE- tazarotene cream
- NDC Code(s): 73503-007-00, 73503-007-01
- Packager: PSODEX USA INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Tazarodex Cream Plaque Psoriasis & Acne
Psoriasis is a chronic disease that often comes and goes.
Acne is a common skin condition that can fluctuate over time.The main goal of treatment is to manage symptoms.
PSODEX Tazarodex Cream offers a soothing formula that helps block the recurrence of plaque psoriasis & acne vulgaris symptoms with the effect of SALICYLIC ACID, UREA, and TAZARATONE.
The main goal of treatment is to manage symptoms.
PREVENTS:
Excessive skin cell growth
RELIEVES:
Itching, inflammation, and roughness
CONTROLS:Flaking, scaling, and excessive sebum secretion
HELPS BLOCK:
Recurrence of psoriasis and acne symptoms -
Tazarodex Cream Plaque Psoriasis & Acne
Inactive Ingredients:
Aloe Barbadensis Leaf Extract, Allantoin, a-Bisabolol,Benzoic Acid, Cetyl Alcohol, Dehydroacetic Acid, Di-C12-13 Alkyl Malate, Dipropylene Glycol, Distilled Water, Glyceryl Monostearate, Laureth-9, Mentha Piperita Extract, Octyldodecanol, Panthenol, Paraffinum Liquidum, Phenoxyethanol, Salicylic Acid, Urea, Xanthan Gum
- Tazarodex Cream Plaque Psoriasis & Acne
- Tazarodex Cream Plaque Psoriasis & Acne
-
Tazarodex Cream Plaque Psoriasis & Acne - Directions
Directions:
Use on affected areas. Make sure the affected area is dry before use. If you are using a moisturizer, use Tazarodex Cream at least
1 hour after applying the moisturizer to the affected area. Avoid sun exposure after applying Tazarodex Cream as it will make your
skin more sensitive to UV rays for 6 hours. Spread it evenly and avoid excessive application. For best results, use only nights or as
directed by a doctor. - Tazarodex Cream Plaque Psoriasis & Acne
- Tazarodex Cream Plaque Psoriasis & Acne
-
Tazarodex Cream Plaque Psoriasis & Acne
Do not use:
- On children under 12 years of age, pregnant or breastfeeding women, or individuals with eczema or retinoid hypersensitivity.
- For prolonged periods of time without consulting a doctor.
- In or around the rectum, genital area, or on the groin unless advised by a doctor.
- On damaged or irritated skin not caused by acne or psoriasis
- Tazarodex Cream Plaque Psoriasis & Acne
- Tazarodex Cream Plaque Psoriasis & Acne
- Tazarodex Cream Plaque Psoriasis & Acne
- Tazarodex Cream Plaque Psoriasis & Acne
- Tazarodex Cream Plaque Psoriasis & Acne
- Tazarodex Cream Plaque Psoriasis & Acne
- Tazarodex Cream Plaque Psoriasis & Acne
- Tazarodex Cream Plaque Psoriasis & Acne
- Tazarodex Cream Plaque Psoriasis & Acne
-
INGREDIENTS AND APPEARANCE
TAZARODEX PLAQUE PSORIASIS AND ACNE
tazarotene creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73503-007 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TAZAROTENE (UNII: 81BDR9Y8PS) (TAZAROTENE - UNII:81BDR9Y8PS) TAZAROTENE 0.1 g in 100 mL Inactive Ingredients Ingredient Name Strength SALICYLIC ACID (UNII: O414PZ4LPZ) 1 g in 100 mL DEHYDROACETIC ACID (UNII: 2KAG279R6R) 0.25 g in 100 mL DI-C12-13 ALKYL MALATE (UNII: RRL7C51WPD) 2 g in 100 mL MINERAL OIL (UNII: T5L8T28FGP) 5 g in 100 mL CETYL ALCOHOL (UNII: 936JST6JCN) 5 g in 100 mL XANTHAN GUM (UNII: TTV12P4NEE) 0.3 g in 100 mL ALLANTOIN (UNII: 344S277G0Z) 2 g in 100 mL POLIDOCANOL (UNII: 0AWH8BFG9A) 3 g in 100 mL MENTHA PIPERITA LEAF (UNII: A389O33LX6) 5 g in 100 mL DIPROPYLENE GLYCOL (UNII: E107L85C40) 0.2 g in 100 mL GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) 2 g in 100 mL PANTHENOL (UNII: WV9CM0O67Z) 1 g in 100 mL BENZOIC ACID (UNII: 8SKN0B0MIM) 0.25 g in 100 mL OCTYLDODECANOL (UNII: 461N1O614Y) 2 g in 100 mL PHENOXYETHANOL (UNII: HIE492ZZ3T) 0.5 g in 100 mL UREA (UNII: 8W8T17847W) 5 g in 100 mL LEVOMENOL (UNII: 24WE03BX2T) 2 g in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73503-007-01 1000 in 1 BOX 01/01/2024 1 NDC:73503-007-00 1000 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M032 01/01/2024 Labeler - PSODEX USA INC (076051073) Registrant - PSODEX USA INC (076051073) Establishment Name Address ID/FEI Business Operations BERKO ILAC VE KIMYA SANAYI ANONIM SIRKETI-SULTANBEYLI SUBESI 533135007 manufacture(73503-007)