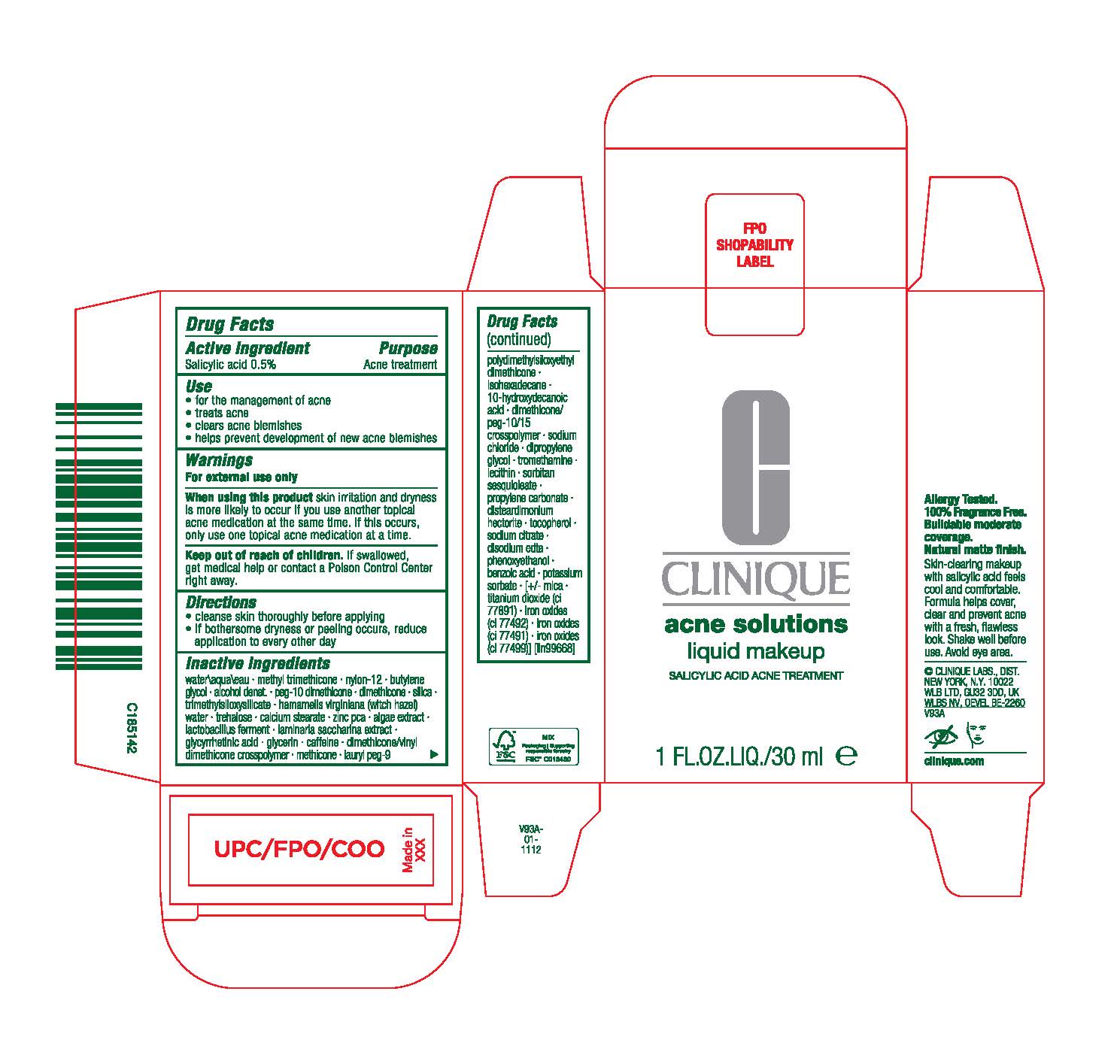
Label: EVEN BETTER CLINICAL ACNE SOLUTIONS- salicylic acid liquid
- NDC Code(s): 49527-114-01
- Packager: CLINIQUE LABORATORIES LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 8, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- USE
- Warnings
- Directions
-
Inactive ingredients
water\aqua\eau· methyl lrimethicone· nylon-12· butylene glycol · aloohol denat.· peg-10 dimelhicme ·dimelhioone· silica· trimelhylsiloxysilicale · hamamelis virginiana(witchhazel) water · trnhalose·calcium stearate · zinc r:ca · algae extract · lactobacillusferment·laminaria saccharina extract·glycyrrhetinicacid · glycerin· caffeine·dimethicone/vinyl dimethiconeCflliSilllymer· melhioone ·lauryl peg-9 polydimethylsiloxyethyl dimethicone ·isohexadecane ·10-hyoroxydecanoic acid· dimethicone/ peg-10/15 crosspolymer· sodium chloride· dipropylene glycol· tromethamine · lecithin·sorbitan sesquioleate · propylene cartrnate· disteardimonium hectonte · tocopherol· sodium citrate· diso:Jiumedta· phenoxyethanol · oonzoicacid· potassium sorbate · [+/-mica· titaniumdioxide(ci 77891) · ironoxides(ci 77492) · iron oxides (ci 77491) · iron oxides (ci 77499)] [iln99668]
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
EVEN BETTER CLINICAL ACNE SOLUTIONS
salicylic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49527-114 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) METHYL TRIMETHICONE (UNII: S73ZQI0GXM) ALCOHOL (UNII: 3K9958V90M) TREHALOSE (UNII: B8WCK70T7I) CALCIUM STEARATE (UNII: 776XM7047L) ZINC PIDOLATE (UNII: C32PQ86DH4) LIMOSILACTOBACILLUS REUTERI (UNII: 9913I24QEE) CAFFEINE (UNII: 3G6A5W338E) ISOHEXADECANE (UNII: 918X1OUF1E) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) SACCHARINA LATISSIMA (UNII: 68CMP2MB55) 10-HYDROXYDECANOIC ACID (UNII: NP03XO416B) DIMETHICONE (UNII: 92RU3N3Y1O) PROPYLENE CARBONATE (UNII: 8D08K3S51E) DIPROPYLENE GLYCOL (UNII: E107L85C40) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) MICA (UNII: V8A1AW0880) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) PHENOXYETHANOL (UNII: HIE492ZZ3T) BENZOIC ACID (UNII: 8SKN0B0MIM) SORBITAN SESQUIOLEATE (UNII: 0W8RRI5W5A) NYLON-12 (UNII: 446U8J075B) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) HAMAMELIS VIRGINIANA TOP WATER (UNII: NT00Y05A2V) PORPHYRIDIUM PURPUREUM (UNII: K2P8K2558N) ENOXOLONE (UNII: P540XA09DR) GLYCERIN (UNII: PDC6A3C0OX) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (HARD PARTICLE) (UNII: H895X08VNQ) METHICONE (20 CST) (UNII: 6777U11MKT) LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: 25G622K2RA) DIMETHICONE/PEG-10/15 CROSSPOLYMER (UNII: 21AS8B1BSS) SODIUM CHLORIDE (UNII: 451W47IQ8X) TROMETHAMINE (UNII: 023C2WHX2V) TOCOPHEROL (UNII: R0ZB2556P8) SODIUM CITRATE (UNII: 1Q73Q2JULR) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49527-114-01 1 in 1 CARTON 09/08/2023 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M006 09/08/2023 Labeler - CLINIQUE LABORATORIES LLC (044475127) Registrant - Estee Lauder Companies Inc. (790802086) Establishment Name Address ID/FEI Business Operations Estee Lauder N.V. 370151326 manufacture(49527-114) , label(49527-114) , pack(49527-114)