Label: POTASSIUM CHLORIDE IN DEXTROSE- dextrose monohydrate and potassium chloride injection, solution injection, solution
- NDC Code(s): 0990-7905-09
- Packager: ICU Medical Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated November 10, 2020
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Potassium Chloride in Dextrose Injection, USP is a sterile and nonpyrogenic solution in water for injection. This solution is for administration by intravenous infusion only.
See Table for summary of content and characteristics of this solution.
This solution contains no bacteriostat, antimicrobial agent or added buffer and is intended only for use as a single-dose injection. When smaller doses are required the unused portion should be discarded.
This solution is a parenteral fluid, nutrient and electrolyte replenisher.
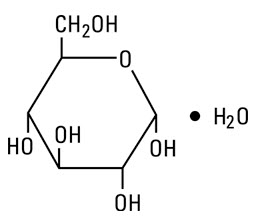
Dextrose, USP is chemically designated D-glucose monohydrate (C6H12O6 ∙ H2O), a hexose sugar freely soluble in water. It has the following structural formula:
Potassium Chloride, USP is chemically designated KCl, a white granular powder freely soluble in water.
Water for Injection, USP is chemically designated H2O.
The flexible plastic container is fabricated from a specially formulated polyvinyl chloride. Water can permeate from inside the container into the overwrap but not in amounts sufficient to affect the solution significantly. Solutions in contact with the plastic container may leach out certain chemical components from the plastic in very small amounts; however, biological testing was supportive of the safety of the plastic container materials. Exposure to temperatures above 25°C/77°F during transport and storage will lead to minor losses in moisture content. Higher temperatures lead to greater losses. It is unlikely that these minor losses will lead to clinically significant changes within the expiration period.
-
CLINICAL PHARMACOLOGY
When administered intravenously, this solution provides a source of water and potassium chloride with carbohydrate.
Solutions containing carbohydrate in the form of dextrose restore blood glucose levels and provide calories. Carbohydrate in the form of dextrose may aid in minimizing liver glycogen depletion and exerts a protein-sparing action. Dextrose injected parenterally undergoes oxidation to carbon dioxide and water.
Intravenous solutions containing potassium chloride are particularly intended to provide needed potassium cation (K+). Potassium is the chief cation of body cells (160 mEq/liter of intracellular water). It is found in low concentration in plasma and extracellular fluids (3.5 to 5.0 mEq/liter in a healthy adult). Potassium plays an important role in electrolyte balance. Normally about 80 to 90% of the potassium intake is excreted in the urine; the remainder in the stools and to a small extent, in the perspiration. The kidney does not conserve potassium well so that during fasting or in patients on a potassium-free diet, potassium loss from the body continues resulting in potassium depletion. A deficiency of either potassium, or chloride will lead to a deficit of the other.
Water is an essential constituent of all body tissues and accounts for approximately 70% of total body weight. Average normal adult daily requirement ranges from two to three liters (1.0 to 1.5 liters each for insensible water loss by perspiration and urine production).
Water balance is maintained by various regulatory mechanisms. Water distribution depends primarily on the concentration of electrolytes in the body compartments and sodium (Na+) plays a major role in maintaining physiologic equilibrium.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
Solutions which contain potassium ions should be used with great care, if at all, in patients with hyperkalemia, severe renal failure and in conditions in which potassium retention is present.
To avoid potassium intoxication, do not infuse these solutions rapidly. In patients with severe renal insufficiency or adrenal insufficiency, administration of potassium chloride may cause potassium intoxication.
In patients with diminished renal function, administration of solutions containing potassium ions may result in potassium retention.
The intravenous administration of this solution can cause fluid and/or solute overloading resulting in dilution of serum electrolyte concentrations, overhydration, congested states or pulmonary edema.
The risk of dilutional states is inversely proportional to the electrolyte concentration of administered parenteral solutions. The risk of solute overload causing congested states with peripheral and pulmonary edema is directly proportional to the electrolyte concentrations of such solutions.
-
PRECAUTIONS
Clinical evaluation and periodic laboratory determinations are necessary to monitor changes in fluid balance, electrolyte concentrations and acid-base balance during prolonged parenteral therapy or whenever the condition of the patient warrants such evaluation.
Solutions containing dextrose should be used with caution in patients with known subclinical or overt diabetes mellitus.
Caution must be exercised in the administration of parenteral fluids, especially those containing sodium ions, to patients receiving corticosteroids or corticotropin.
Potassium replacement therapy should be guided primarily by serial electrocardiograms. Plasma potassium levels are not necessarily indicative of tissue potassium levels.
High plasma concentrations of potassium may cause death through cardiac depression, arrhythmias or arrest.
Potassium-containing solutions should be used with caution in the presence of cardiac disease, particularly in digitalized patients or in the presence of renal disease.
Care should be exercised to insure that the needle (or catheter) is well within the lumen of the vein and that extravasation does not occur.
Do not administer unless solution is clear and container is undamaged. Discard unused portion.
Pregnancy Category C
Animal reproduction studies have not been conducted with dextrose or potassium chloride. It is also not known whether dextrose or potassium chloride can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Dextrose or potassium chloride should be given to a pregnant woman only if clearly needed.
Pediatric use
The safety and effectiveness in the pediatric population are based on the similarity of the clinical conditions of the pediatric and adult populations. In neonates or very small infants the volume of fluid may affect fluid and electrolyte balance.
Frequent monitoring of serum glucose concentrations is required when dextrose is prescribed to pediatric patients, particularly neonates and low birth weight infants.
In very low birth weight infants, excessive or rapid administration of dextrose injection may result in increased serum osmolality and possible intracerebral hemorrhage.
-
ADVERSE REACTIONS
Reactions which may occur because of the solutions or technique of administration include febrile response, infection at the site of injection, venous thrombosis or phlebitis extending from the site of injection, extravasation and hypervolemia.
If an adverse reaction does occur, discontinue the infusion, evaluate the patient, institute appropriate therapeutic countermeasures and save the remainder of the fluid for examination if deemed necessary.
Nausea, vomiting, abdominal pain and diarrhea have been reported with potassium therapy. The signs and symptoms of potassium intoxication include paresthesias of the extremities, flaccid paralysis, listlessness, mental confusion, weakness and heaviness of the legs, hypotension, cardiac arrhythmias, heart block, electrocardiographic abnormalities such as disappearance of P waves, spreading and slurring of the QRS complex with development of a biphasic curve and cardiac arrest.
Potassium-containing solutions are intrinsically irritating to tissues. Therefore, extreme care should be taken to avoid perivascular infiltration. Local tissue necrosis and subsequent sloughing may result if extravasation occurs. Chemical phlebitis and venospasm have also been reported.
Should perivascular infiltration occur, I.V. administration at that site should be discontinued at once. Local infiltration of the affected area with procaine hydrochloride, 1%, to which hyaluronidase may be added, will often reduce venospasm and dilute the potassium remaining in the tissues locally. Local application of heat may also be helpful.
-
OVERDOSAGE
In the event of potassium overdosage, discontinue the infusion immediately and institute intensive corrective therapy to reduce serum potassium levels. See WARNINGS and PRECAUTIONS.
-
DOSAGE AND ADMINISTRATION
This solution should be administered only by intravenous infusion and as directed by the physician. The dose and rate of injection are dependent upon the age, weight and clinical condition of the patient. If the serum potassium level is greater than 2.5 mEq/liter, potassium should be given at a rate not to exceed 10 mEq/hour in a concentration less than 30 mEq/liter. Somewhat faster rates and greater concentrations (usually up to 40 mEq/liter) of potassium may be indicated in patients with more severe potassium deficiency. The total 24-hour dose should not generally exceed 200 mEq of potassium.
As reported in the literature, the dosage and constant infusion rate of intravenous dextrose must be selected with caution in pediatric patients, particularly neonates and low birth weight infants, because of the increased risk of hyperglycemia/hypoglycemia.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. See PRECAUTIONS.
-
INSTRUCTIONS FOR USE
To Open
Tear outer wrap at notch and remove solution container. If supplemental medication is desired, follow directions below before preparing for administration. Some opacity of the plastic due to moisture absorption during the sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually.
To Add Medication
- Prepare additive port.
- Using aseptic technique and an additive delivery needle of appropriate length, puncture resealable additive port at target area, inner diaphragm and inject. Withdraw needle after injecting medication.
- The additive port may be protected by covering with an additive cap.
- Mix container contents thoroughly.
Preparation for Administration
(Use aseptic technique)- Close flow control clamp of administration set.
- Remove cover from outlet port at bottom of container.
- Insert piercing pin of administration set into port with a twisting motion until the set is firmly seated. NOTE: When using a vented administration set, replace bacterial retentive air filter with piercing pin cover. Insert piercing pin with twisting motion until shoulder of air filter housing rests against the outlet port flange.
- Suspend container from hanger.
- Squeeze and release drip chamber to establish proper fluid level in chamber.
- Attach venipuncture device to set.
- Open clamp to expel air from set and venipuncture device. Close clamp.
- Perform venipuncture.
- Regulate rate of administration with flow control clamp.
WARNING: Do not use flexible container in series connections.
-
HOW SUPPLIED
Potassium Chloride in Dextrose Injection, USP solution is supplied in single-dose flexible plastic containers. See Table:
Table of Contents and Characteristics Grams/100 mL Per 1000 mL mEq
PotassiumSize
(mL)NDC No. Dextrose Hydrous Potassium Chloride Potassium
(K+)Chloride
(Cl¯)Caloric Value Calculated Osmolarity
(mOsmol)Tonicity pH 20 mEq 1000 0409–7905–09 5 0.149 20 mEq 20 mEq 170 292 isotonic 4.3 (3.5 to 6.5) 20 mEq 1000 0990–7905–09 5 0.149 20 mEq 20 mEq 170 292 isotonic 4.3 (3.5 to 6.5) ICU Medical is transitioning NDC codes from the "0409" to "0990" labeler code. Both NDC codes are expected to be in the market for a period of time.
May contain HCl for pH adjustment.
- SPL UNCLASSIFIED SECTION
-
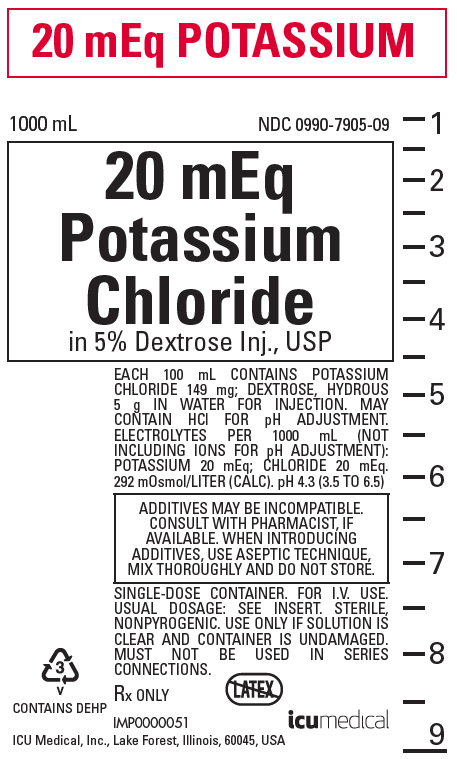
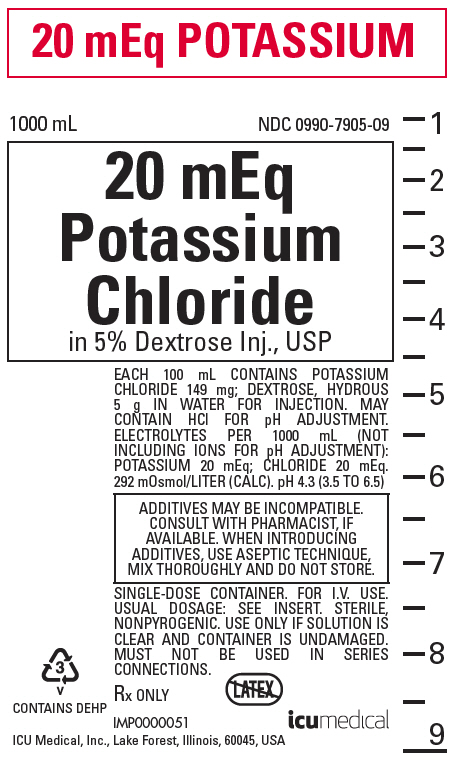
PRINCIPAL DISPLAY PANEL - 1000 mL Bag Label
20 mEq POTASSIUM
1000 mL
NDC 0990-7905-0920 mEq
Potassium
Chloride
in 5% Dextrose Inj., USPEACH 100 mL CONTAINS POTASSIUM
CHLORIDE 149 mg; DEXTROSE, HYDROUS
5 g IN WATER FOR INJECTION. MAY
CONTAIN HCl FOR pH ADJUSTMENT.
ELECTROLYTES PER 1000 mL (NOT
INCLUDING IONS FOR pH ADJUSTMENT):
POTASSIUM 20 mEq; CHLORIDE 20 mEq.
292 mOsmol/LITER (CALC). pH 4.3 (3.5 TO 6.5)ADDITIVES MAY BE INCOMPATIBLE.
CONSULT WITH PHARMACIST, IF
AVAILABLE. WHEN INTRODUCING
ADDITIVES, USE ASEPTIC TECHNIQUE,
MIX THOROUGHLY AND DO NOT STORE.SINGLE-DOSE CONTAINER. FOR I.V. USE.
USUAL DOSAGE: SEE INSERT. STERILE,
NONPYROGENIC. USE ONLY IF SOLUTION IS
CLEAR AND CONTAINER IS UNDAMAGED.
MUST NOT BE USED IN SERIES
CONNECTIONS.Rx ONLY
3
v
CONTAINS DEHPIMP0000051
ICU Medical, Inc., Lake Forest, Illinois, 60045, USAicumedical
-
INGREDIENTS AND APPEARANCE
POTASSIUM CHLORIDE IN DEXTROSE
dextrose monohydrate and potassium chloride injection, solution injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0990-7905 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) DEXTROSE MONOHYDRATE 5 g in 100 mL POTASSIUM CHLORIDE (UNII: 660YQ98I10) (POTASSIUM CATION - UNII:295O53K152, CHLORIDE ION - UNII:Q32ZN48698) POTASSIUM CHLORIDE 0.149 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0990-7905-09 12 in 1 CASE 11/20/2020 1 1 in 1 POUCH 1 1000 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA018371 11/20/2020 Labeler - ICU Medical Inc. (118380146)