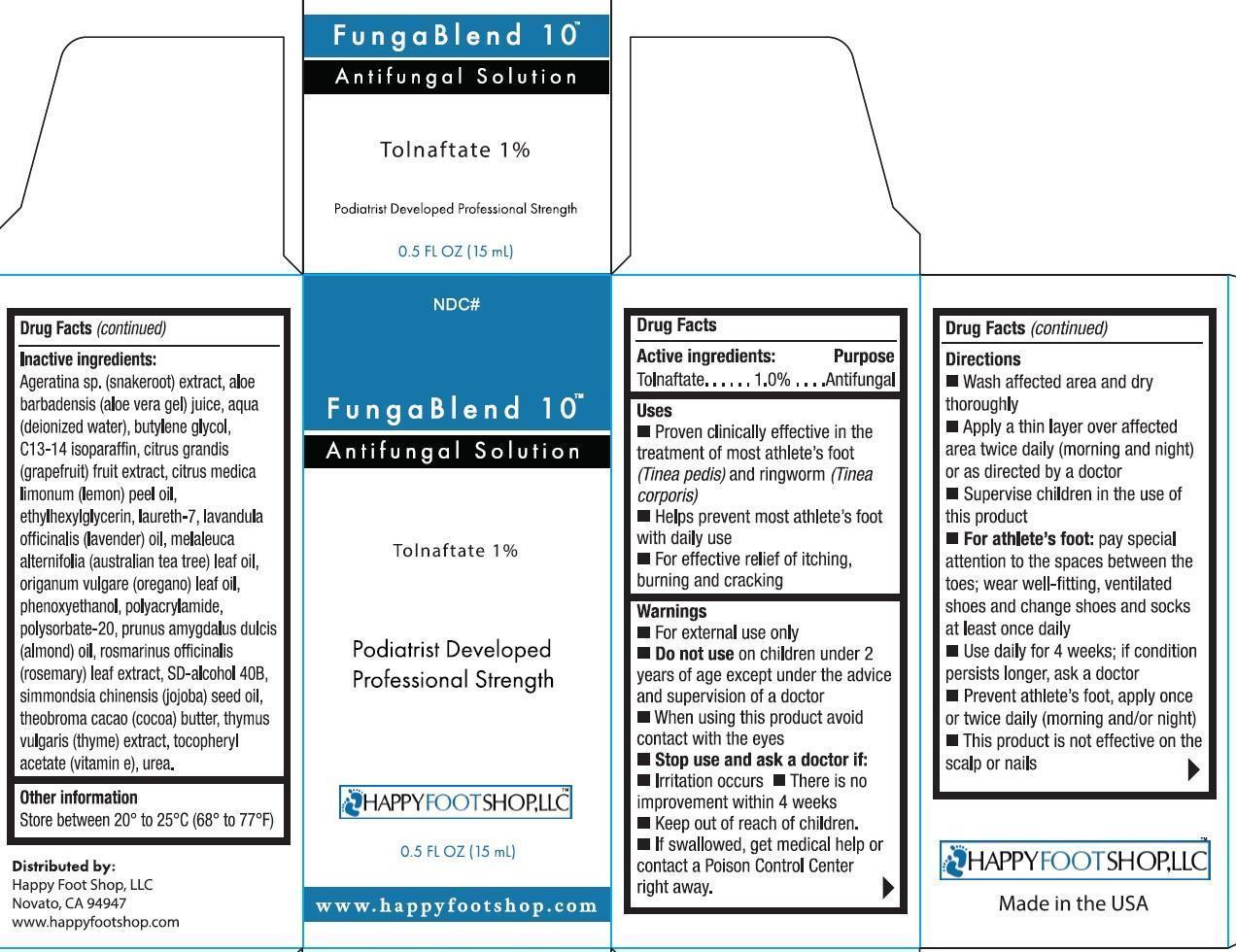
Label: FUNGABLEND 10- tolnaftate liquid
- NDC Code(s): 69983-026-01
- Packager: Happy Foot Shop, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients:
- Uses
- Warnings
-
Directions
- Wash affected area and dry thoroughly
- Apply a thin layer over affected area twice daily (morning and night) or as directed by a doctor
- Supervise children in the use of this product
- For athlete's foot: pay special attention to the spaces between the toes; wear well-fitting, ventilated shoes and change shoes and socks at least once daily.
- Use daily for 4 weeks; if condition persists longer, ask a doctor
- Prevent athlete's foot, apply once or twice daily (morning and/or night)
- This product is not effective on scalp or nails
-
Inactive ingredients:
Ageratina sp. (snakeroot) extract, aloe barbadensis (aloe vera gel) juice, aqua (deionized water), butylene glycol, C13-14 isoparaffin, citrus grandis (grapefruit) fruit extract, citrus medica limonum (lemon) peel oil, ethylhexylglycerin, laureth-7, lavandula officinalis (lavender) oil, melaleuca alternifolia (australia tea tree) leaf oil, origanum vulgare (oregano) leaf oil, phenoxyethanol, polyacrylamide, polysorbate-20, prunus amygdalus dulcis (almond) oil, rosmarinus officinalis (rosemary) leaf extract, SD-alcohol 40B, simmondsia chinensis (jojoba) seed oil, theobroma cacao (cocoa) butter, thymus vulgaris (thyme) extract, tocopheryl acetate (vitamin e), urea.
- Other information
- Product Label
-
INGREDIENTS AND APPEARANCE
FUNGABLEND 10
tolnaftate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69983-026 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TOLNAFTATE (UNII: 06KB629TKV) (TOLNAFTATE - UNII:06KB629TKV) TOLNAFTATE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) WATER (UNII: 059QF0KO0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) C13-14 ISOPARAFFIN (UNII: E4F12ROE70) PUMMELO (UNII: ET1TN5W71X) LEMON OIL (UNII: I9GRO824LL) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) LAURETH-7 (UNII: Z95S6G8201) LAVENDER OIL (UNII: ZBP1YXW0H8) TEA TREE OIL (UNII: VIF565UC2G) OREGANO LEAF OIL (UNII: 7D0CGR40U1) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYSORBATE 20 (UNII: 7T1F30V5YH) ALMOND OIL (UNII: 66YXD4DKO9) ROSEMARY (UNII: IJ67X351P9) ALCOHOL (UNII: 3K9958V90M) JOJOBA OIL (UNII: 724GKU717M) COCOA BUTTER (UNII: 512OYT1CRR) THYME (UNII: CW657OBU4N) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) UREA (UNII: 8W8T17847W) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69983-026-01 1 in 1 CARTON 02/23/2017 02/23/2017 1 15 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 02/23/2017 Labeler - Happy Foot Shop, LLC (079776150)