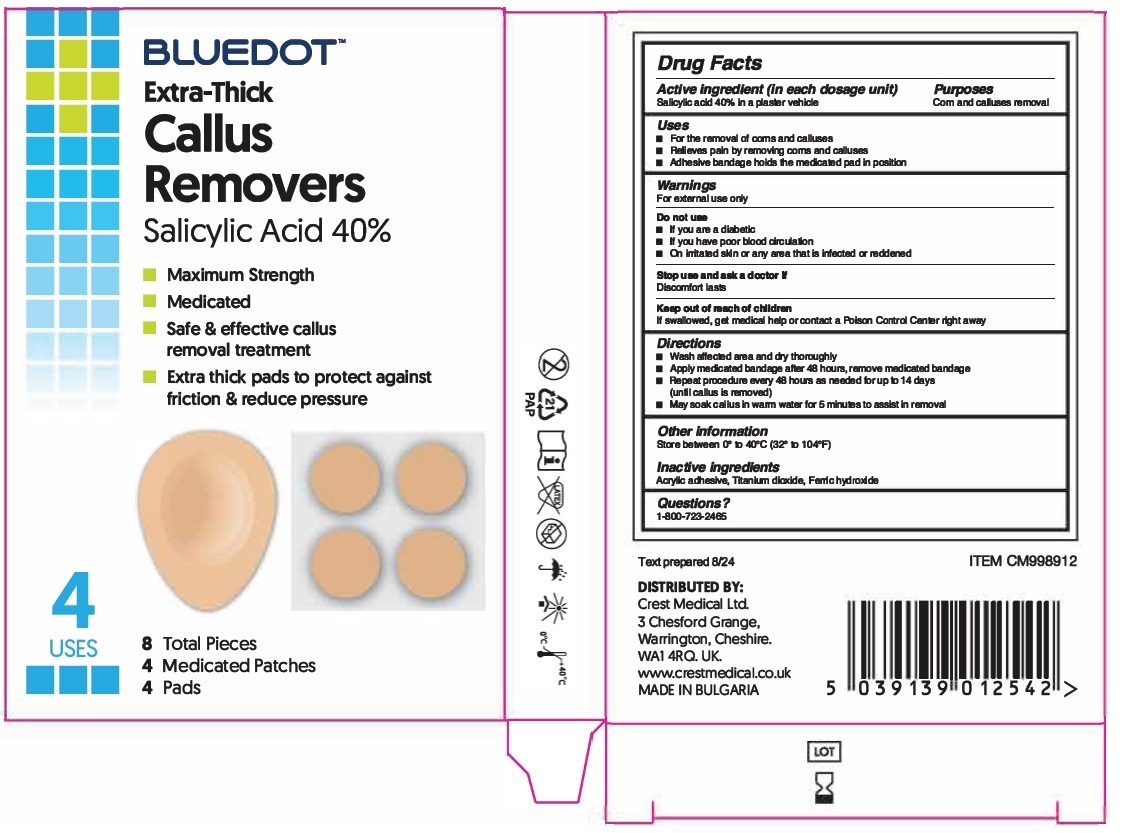
Label: BLUEDOT CALLUS REMOVERS- salicylic acid patch
- NDC Code(s): 84713-103-04
- Packager: CREST MEDICAL LIMITED
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated September 9, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient (in each dosage unit)
- Purposes
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- Packaging
-
INGREDIENTS AND APPEARANCE
BLUEDOT CALLUS REMOVERS
salicylic acid patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84713-103 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 40 g in 100 g Inactive Ingredients Ingredient Name Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC HYDROXIDE (UNII: 2UA751211N) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84713-103-04 1 in 1 BOX 09/15/2024 1 4 in 1 BAG 1 0.0275 g in 1 PATCH; Type 4: Device Coated/Impregnated/Otherwise Combined with Drug Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M030 09/15/2024 Labeler - CREST MEDICAL LIMITED (238858539) Establishment Name Address ID/FEI Business Operations KRE EOOD 565504983 manufacture(84713-103) , label(84713-103) , pack(84713-103)