Label: MULTI-SYMPTOM COLD AND FLU-KIDS- acetaminophen 320 mg, dextromethorphan hbr 5 mg, guaifenesin 100 mg suspension
- NDC Code(s): 69676-0087-9
- Packager: Genexa Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 7, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
-
INDICATIONS & USAGE
Uses
• temporarily relieves:
• cough due to minor throat and bronchial irritation as may occur with the common cold or inhaled irritants
• the intensity of coughing
• the impulse to cough to help your child get to sleep
• minor aches and pains associated with:
• the common cold • fever • sore throat
• headache • body pain
• helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of
bothersome mucus and make coughs more productive
-
WARNINGS
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if your child takes
• more than 5 doses in 24 hours, which is the maximum daily amount
• with other drugs containing acetaminophen
Allergy alert: acetaminophen may cause severe skin reactions. Symptoms may include:
• skin reddening
• blisters
• rash
If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning: if sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
• with any other drug containing acetaminophen (prescription or nonprescription). If you are not
sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
• in a child who is taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for
depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping
the MAOI drug. If you do not know if your child’s prescription drug contains an MAOI, ask a doctor or
pharmacist before giving this product.
Ask a doctor before use if the child has
• Liver disease
• cough that occurs with too much phlegm (mucus)
• persistent or chronic cough such as occurs with asthma or if cough is accompanied by excessive phlegm
(mucus) unless directed by a doctor.
Stop use and ask a doctor if
• pain gets worse or lasts more than 5 days
• fever gets worse or lasts more than 3 days
• new symptoms occur
• redness or swelling is present
• cough persists for more than 1 week, tends to recur, or is accompanied by a fever, rash, or
persistent headache.
These could be signs of a serious condition.
-
DOSAGE & ADMINISTRATION
Directions
• this product does not contain directions or complete warnings for adult use
• do not give more than directed (see Overdose warning)
• SHAKE WELL BEFORE USING
• find right dose on chart below
• for accurate dosing, use measuring cup provided.
Do not use with any other dosing device.
• give dose every 4 hours while symptoms last
• do not give more than 5 times in 24 hours
children 6 to under 12 years 10 mL every 4 hours children 4 to under 6 years 7.5 mL every 4 hours children under 4 years do not use - STORAGE AND HANDLING
- INACTIVE INGREDIENT
- QUESTIONS
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
-
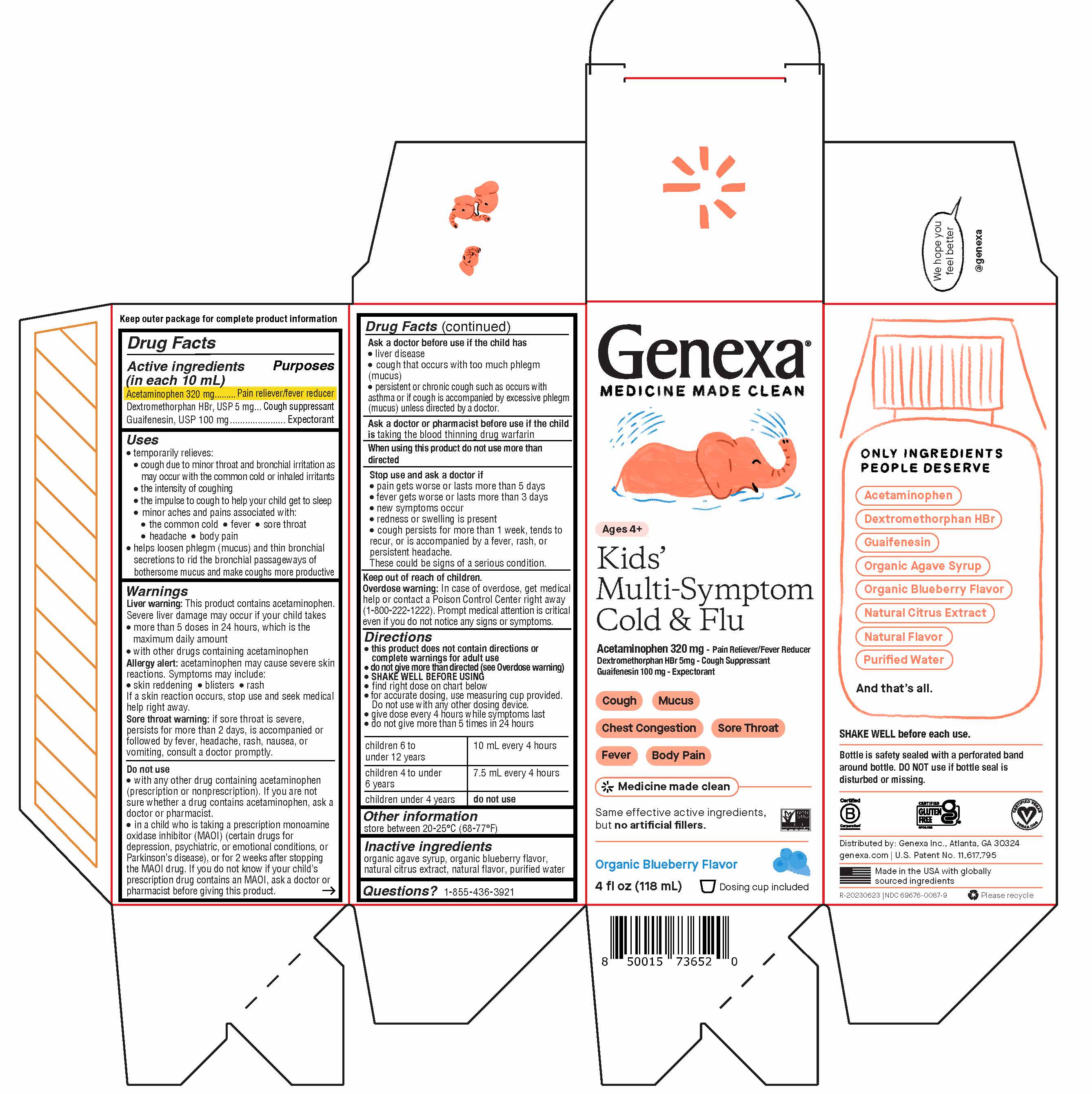
PRINCIPAL DISPLAY PANEL
Genexa®
MEDICINE MADE CLEAN
Ages 4+
Kids' Multi-Symptom Cold & Flu
Acetaminophen 320 mg - Pain Reliever/Fever Reducer
Dextromethorphan HBr 5mg - Cough Suppressant
Guaifenesin 100 mg - Expectorant
Cough
Mucus
Chest Congestion
Sore Throat
Fever
Body Pain
Medicine made clean
Same effective active ingredients, but no artificial fillers.
Organic Blueberry Flavor
4 FL OZ (118 mL) Liquid
Dosing cup included
-
INGREDIENTS AND APPEARANCE
MULTI-SYMPTOM COLD AND FLU-KIDS
acetaminophen 320 mg, dextromethorphan hbr 5 mg, guaifenesin 100 mg suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69676-0087 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 100 mg in 10 mL ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 320 mg in 10 mL DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 5 mg in 10 mL Inactive Ingredients Ingredient Name Strength CITRUS FRUIT (UNII: XDK00Z8012) AGAVE TEQUILANA JUICE (UNII: GVG8G0207O) WATER (UNII: 059QF0KO0R) Product Characteristics Color brown (Gold) Score Shape Size Flavor BLUEBERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69676-0087-9 1 in 1 CARTON 08/15/2023 1 118 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M013 08/15/2023 Labeler - Genexa Inc. (079751024)

