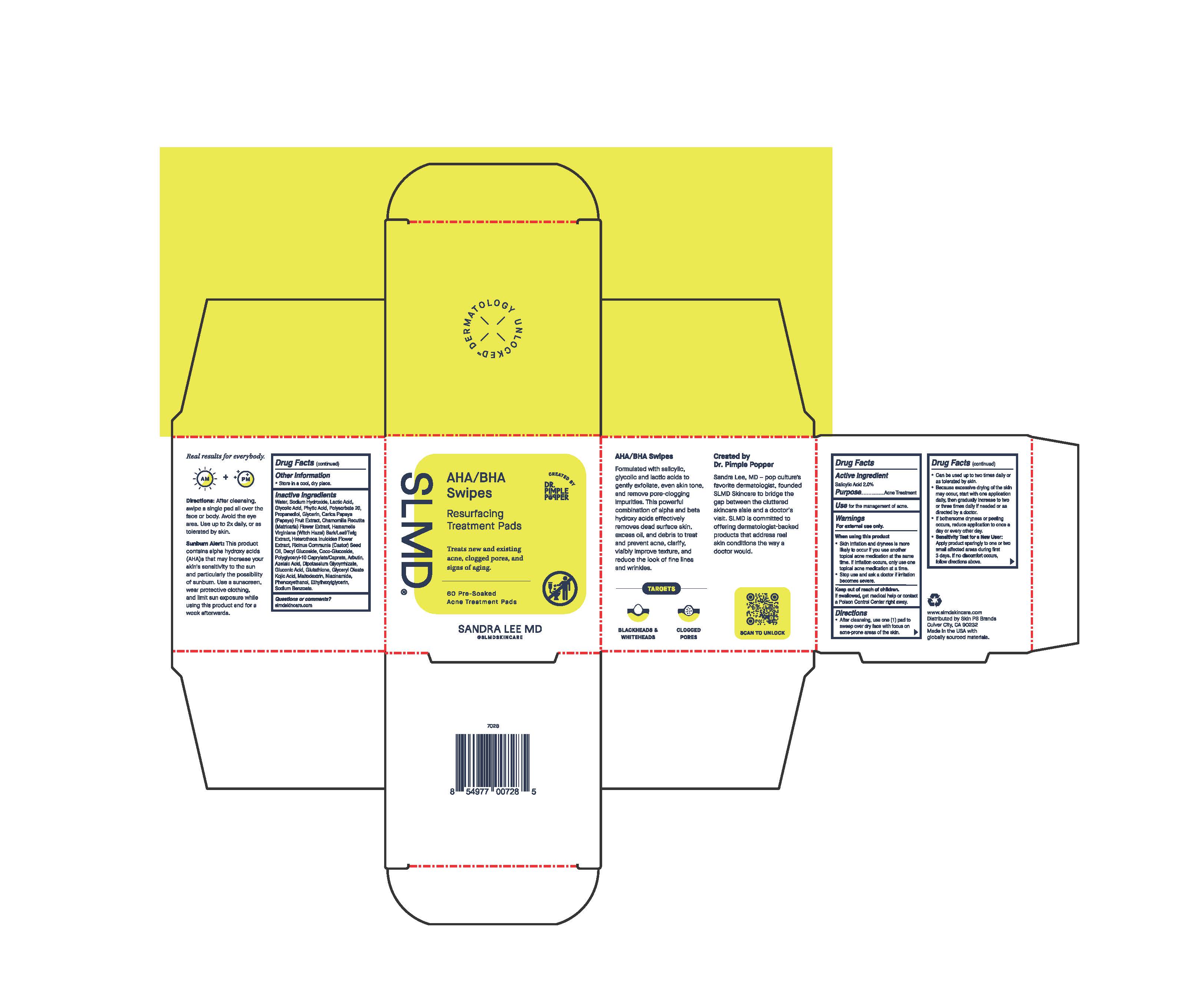
Label: AHA/BHA SWIPES- resurfacing treatment pads swab
- NDC Code(s): 73318-7004-6
- Packager: SKIN PS Brands
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated August 7, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings
-
Directions
• after cleansing, use one (1) pad to sweep
over dry face with focus on acne-prone
areas of the skin
• Can be used up to two times daily or as
tolerated by skin
• Because excessive drying of the skin may
occur, start with one application daily, then
gradually increase to two or three times
daily if needed or as directed by a doctor
• If bothersome dryness or peeling occurs,
reduce application to once a day or every
other day.
• Sensitivity Test for a New User:
apply product sparingly to one or two small
affected areas during first 3 days. If no
discomfort occurs, follow directions above. - Other Information
-
Inactive ingredients
Water, Sodium Hydroxide, Lactic Acid, Glycolic Acid, Phytic Acid, Polysorbate 20, Propanediol, Glycerin, Carica Papaya (Papaya) Fruit Extract, Chamomilla Recutita (Matricaria) Flower Extract, Hamamelis Virginiana (Witch Hazel) Bark/Leaf/Twig Extract, Heterotheca Inuloides Flower Extract, Ricinus Communis (Castor) Seed Oil, Decyl Glucoside, Coco-Glucoside, Polyglyceryl-10 Caprylate/Caprate, Arbutin, Azelaic Acid, Dipotassium Glycyrrhizate, Gluconic Acid, Glutathione, Glyceryl Oleate, Kojic Acid, Maltodextrin, Niacinamide, Phenoxyethanol, Ethylhexylglycerin, Sodium Benzoate.
- Questions or comments?
-
Label
SLMD Created By Dr. Pimple Popper
AHA/BHA Swipes
Resurfacing Treatment Pads
Treats new and existing acne, clogged pores, and signs of aging.
60 Pre-Soaked
Acne Treatment Pads
SANDRA LEE MD
@slmdskincare
AHA/BHA Swipes
Formulated with salicylic, glycolic and lactic acids to gently exfoliate, even skin tone, and remove pore-clogging impurities. This powerful combination of alpha and beta hydroxy acids effectively removes dead surface skin, excess oil, and debris to treat and prevent acne, clarify, visibly improve texture, and reduce the look of fine lines and wrinkles.
TARGETS
BLACKHEADS & WHITEHEADS
CLOGGED PORES
Created by Dr. Pimple Popper
Sandra Lee, MD - pop culture's favorite dermatologist, founded SLMD Skincare to bridge the gap between the cluttered skincare aisle and a doctor's visit. SLMD is committed to offering dermatologist-backed products that address real skin conditions the way a doctor would.
Real results for everybody
Directions: After cleansing, swipe a single pad all over the face or body. Avoid the eye area. Use up to 2x daily, or as tolerated by skin.
Sunburn Alert: This product contains alpha hydroxy acids (AHA)s that may increase your skin's sensitivity to the sun and particularly the possibility of sunburn. Use a sunscreen, wear protective clothing, and limit sun exposure while using this product and for a week afterward
www.slmdskincare.com
Distributed by Skin PS Brands
Culver City, CA 90232
Made in the USA with globally sourced materials.
-
INGREDIENTS AND APPEARANCE
AHA/BHA SWIPES
resurfacing treatment pads swabProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73318-7004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 2 g in 100 mL Inactive Ingredients Ingredient Name Strength POLYGLYCERYL-10 CAPRYLATE (UNII: YS396CQX5C) PHENOXYETHANOL (UNII: HIE492ZZ3T) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) AZELAIC ACID (UNII: F2VW3D43YT) WATER (UNII: 059QF0KO0R) FYTIC ACID (UNII: 7IGF0S7R8I) GLYCYRRHIZINATE DIPOTASSIUM (UNII: CA2Y0FE3FX) DECYL GLUCOSIDE (UNII: Z17H97EA6Y) COCO GLUCOSIDE (UNII: ICS790225B) GLYCERYL OLEATE (UNII: 4PC054V79P) GLUCONIC ACID (UNII: R4R8J0Q44B) SODIUM BENZOATE (UNII: OJ245FE5EU) HETEROTHECA INULOIDES FLOWER (UNII: W9NZ9OZF68) RICINUS COMMUNIS SEED (UNII: 7EK4SFN1TX) SODIUM HYDROXIDE (UNII: 55X04QC32I) GLYCOLIC ACID (UNII: 0WT12SX38S) LACTIC ACID, L- (UNII: F9S9FFU82N) POLYSORBATE 20 (UNII: 7T1F30V5YH) CARICA PAPAYA WHOLE (UNII: S0U63B0Q51) KOJIC ACID (UNII: 6K23F1TT52) NIACINAMIDE (UNII: 25X51I8RD4) PROPANEDIOL (UNII: 5965N8W85T) GLYCERIN (UNII: PDC6A3C0OX) MATRICARIA CHAMOMILLA FLOWERING TOP (UNII: 3VNC7T6Z02) HAMAMELIS VIRGINIANA WHOLE (UNII: V663Q8TEFU) MALTODEXTRIN (UNII: 7CVR7L4A2D) ARBUTIN (UNII: C5INA23HXF) GLUTATHIONE (UNII: GAN16C9B8O) Product Characteristics Color white (white round pads impregnated with topical acne solution) Score Shape ROUND (Round pads) Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73318-7004-6 1 in 1 BOX 07/01/2023 1 60 mL in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M006 07/01/2023 Labeler - SKIN PS Brands (081085221) Registrant - SKIN PS Brands (081085221) Establishment Name Address ID/FEI Business Operations Diamond Wipes International, Inc. 161104729 manufacture(73318-7004)