Label: BARRIER BALM ointment
-
NDC Code(s):
51514-0363-1,
51514-0363-2,
51514-0363-3,
51514-0363-4, view more51514-0363-5, 51514-0363-6
- Packager: Autumn Harp, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated August 1, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
For external use only
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away
Do not use on deep or puncture wounds, animal bites, serious burns
When using this product do not get into eyes
Stop use and ask doctor if condition worsens, symptoms last more than 7 days or clear up and occur again within a few days
- INACTIVE INGREDIENT
- QUESTIONS
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
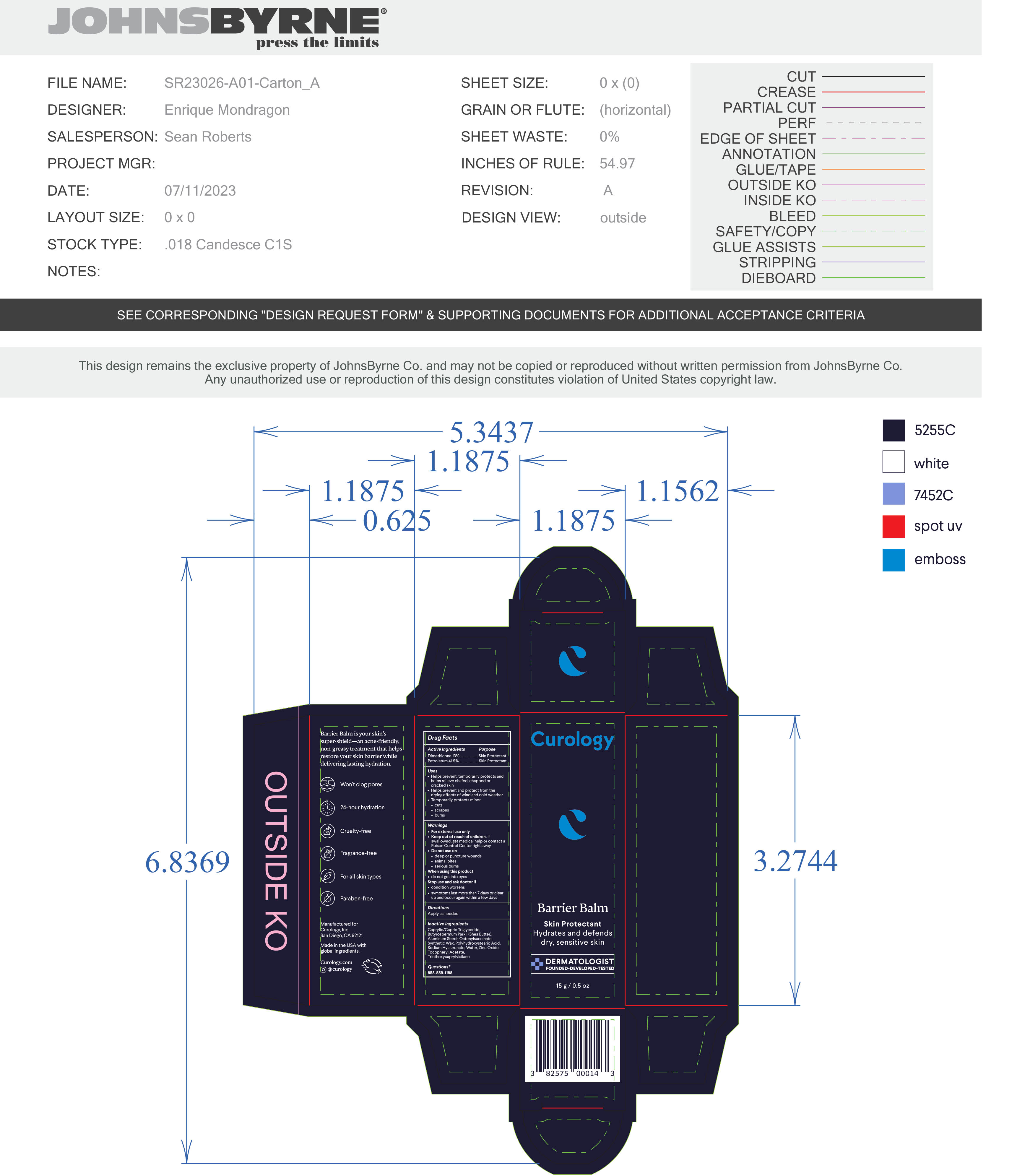
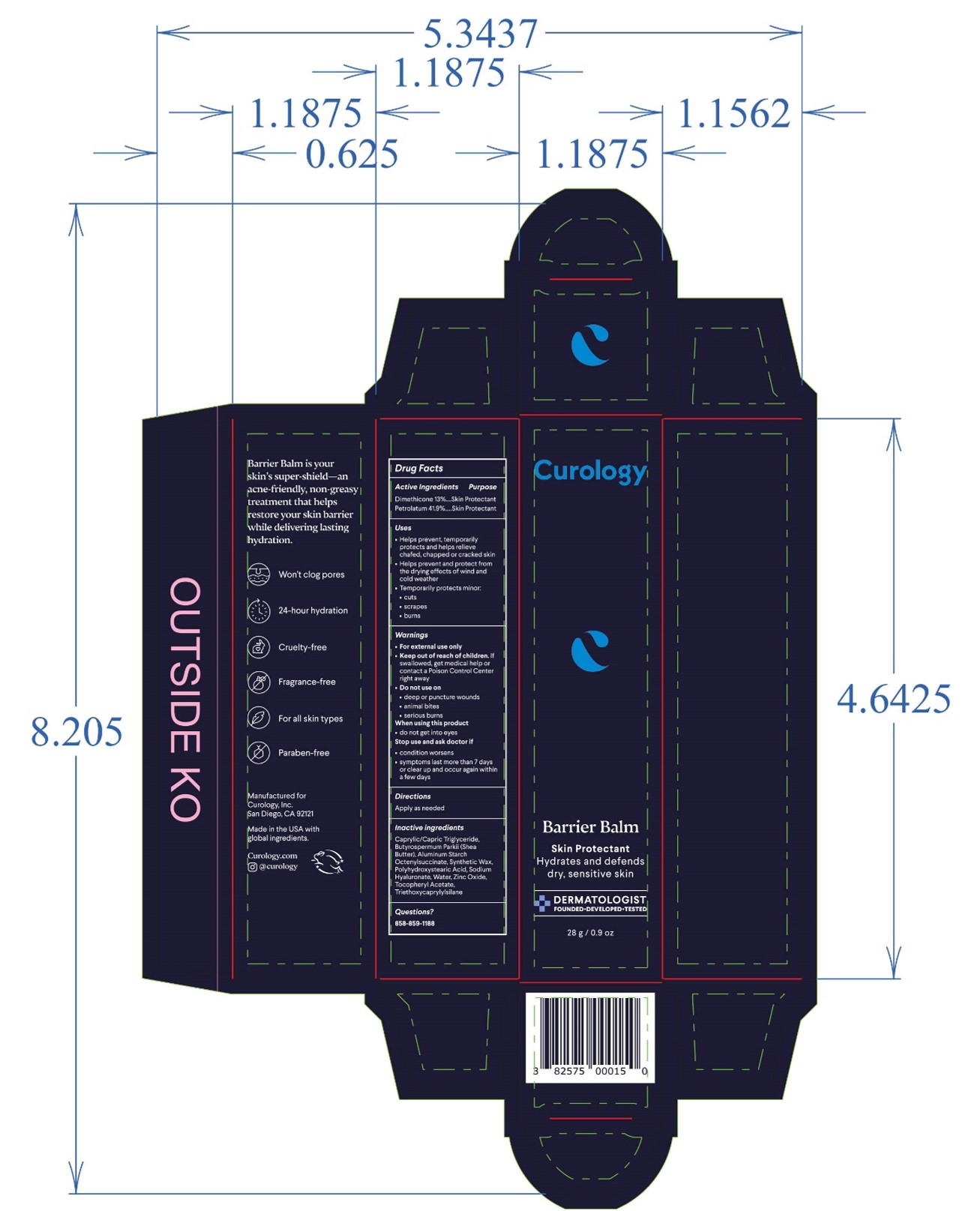
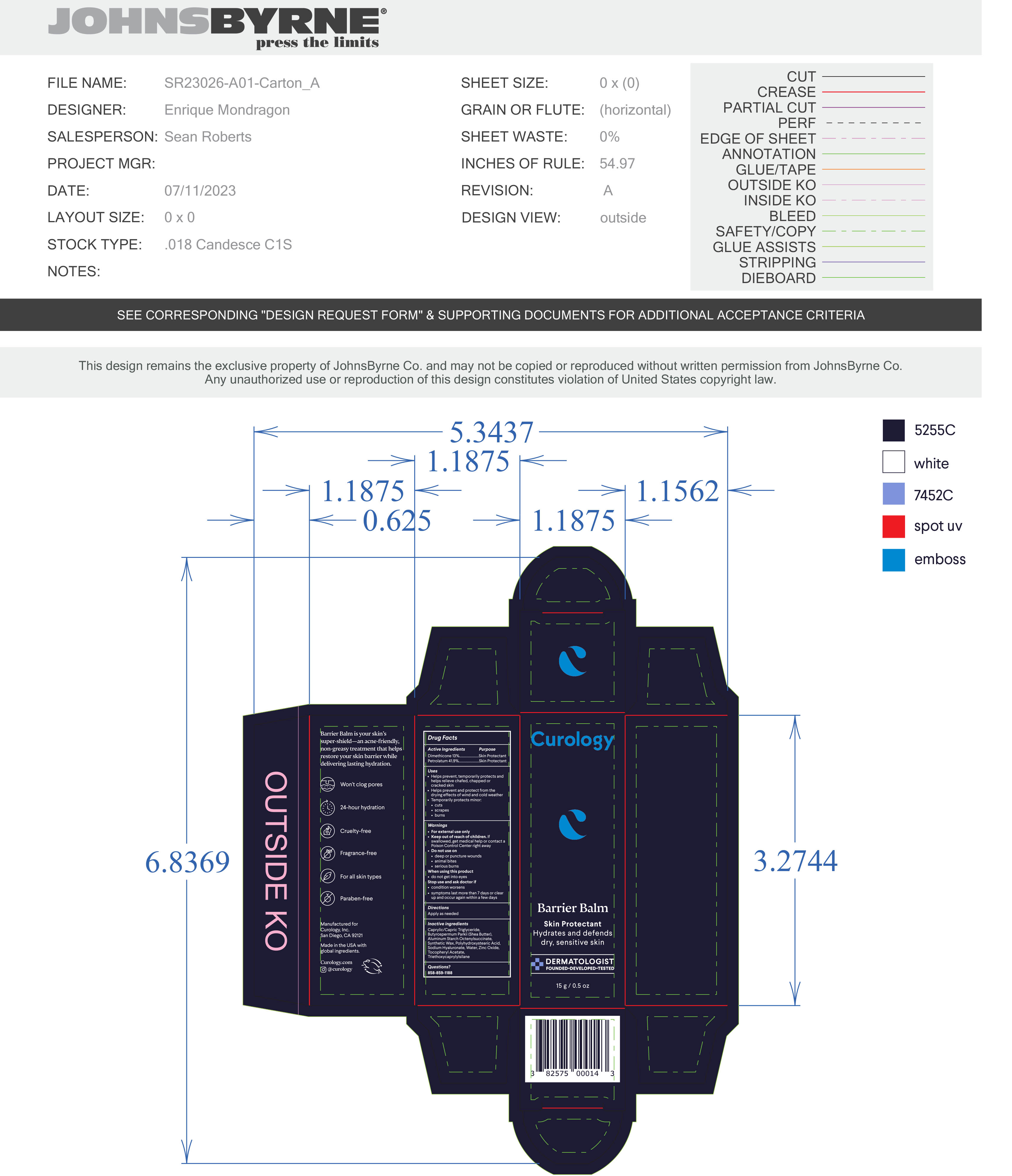
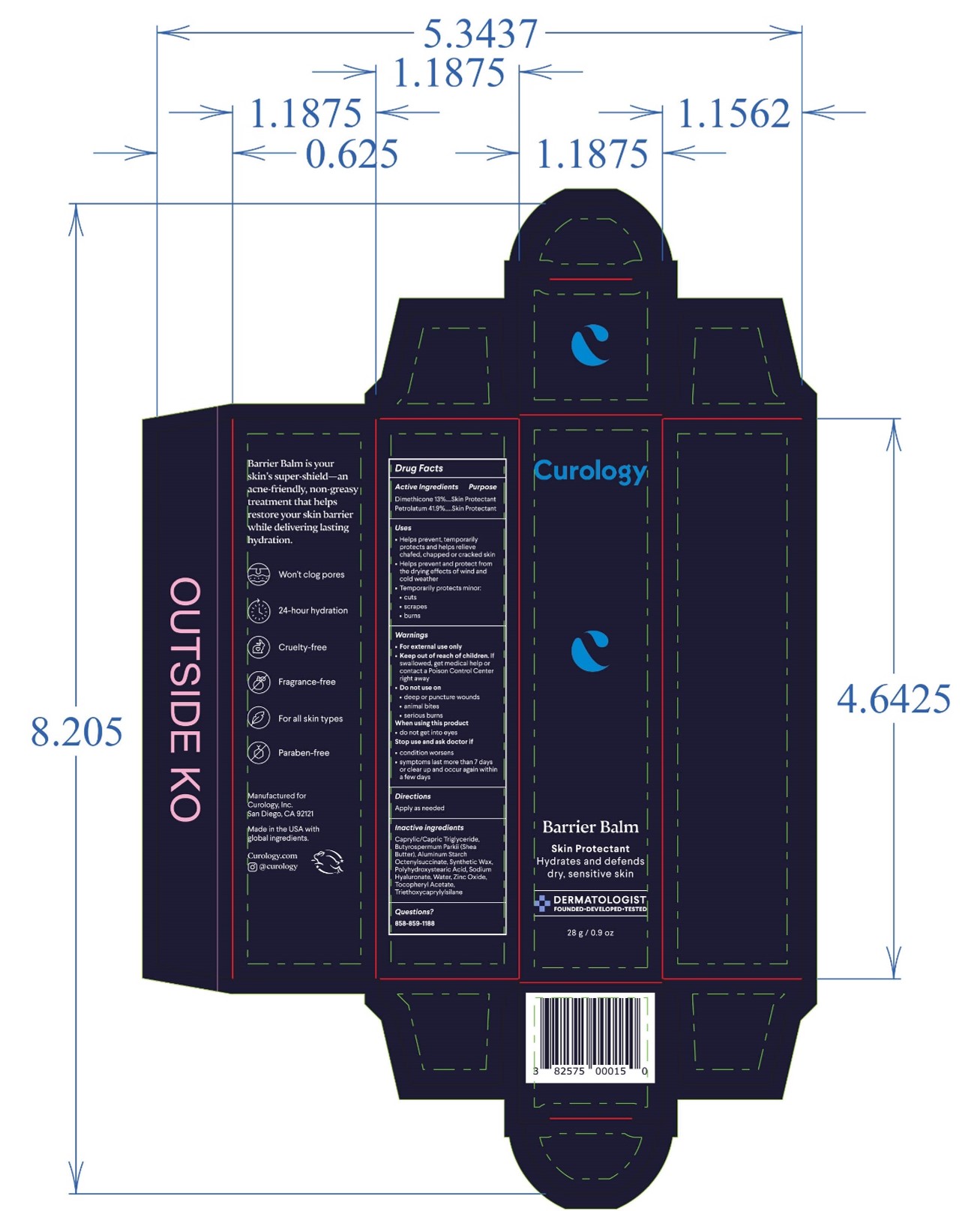
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BARRIER BALM
barrier balm ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51514-0363 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE 20 (UNII: H8YMB5QY0D) (DIMETHICONE 20 - UNII:H8YMB5QY0D) DIMETHICONE 20 13 g in 100 g PETROLATUM (UNII: 4T6H12BN9U) (PETROLATUM - UNII:4T6H12BN9U) PETROLATUM 41.9 g in 100 g Inactive Ingredients Ingredient Name Strength POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) ZINC OXIDE (UNII: SOI2LOH54Z) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) SYNTHETIC WAX (1800 MW) (UNII: 248P1AUJ90) WATER (UNII: 059QF0KO0R) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) SHEA BUTTER (UNII: K49155WL9Y) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) HYALURONATE SODIUM (UNII: YSE9PPT4TH) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51514-0363-2 1 in 1 CARTON 10/02/2023 1 NDC:51514-0363-1 15 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:51514-0363-4 1 in 1 CARTON 10/02/2023 2 NDC:51514-0363-3 28 g in 1 TUBE; Type 0: Not a Combination Product 3 NDC:51514-0363-6 1 in 1 CARTON 10/02/2023 3 NDC:51514-0363-5 48 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 10/02/2023 Labeler - Autumn Harp, Inc. (064187883) Establishment Name Address ID/FEI Business Operations Autumn Harp 064187883 manufacture(51514-0363)