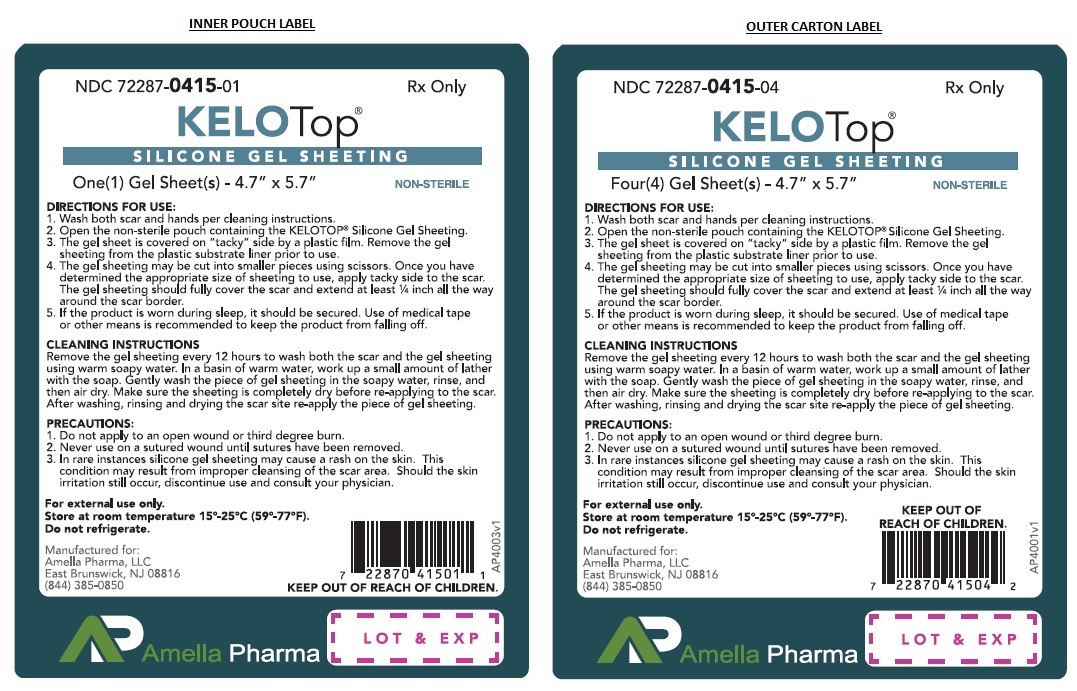
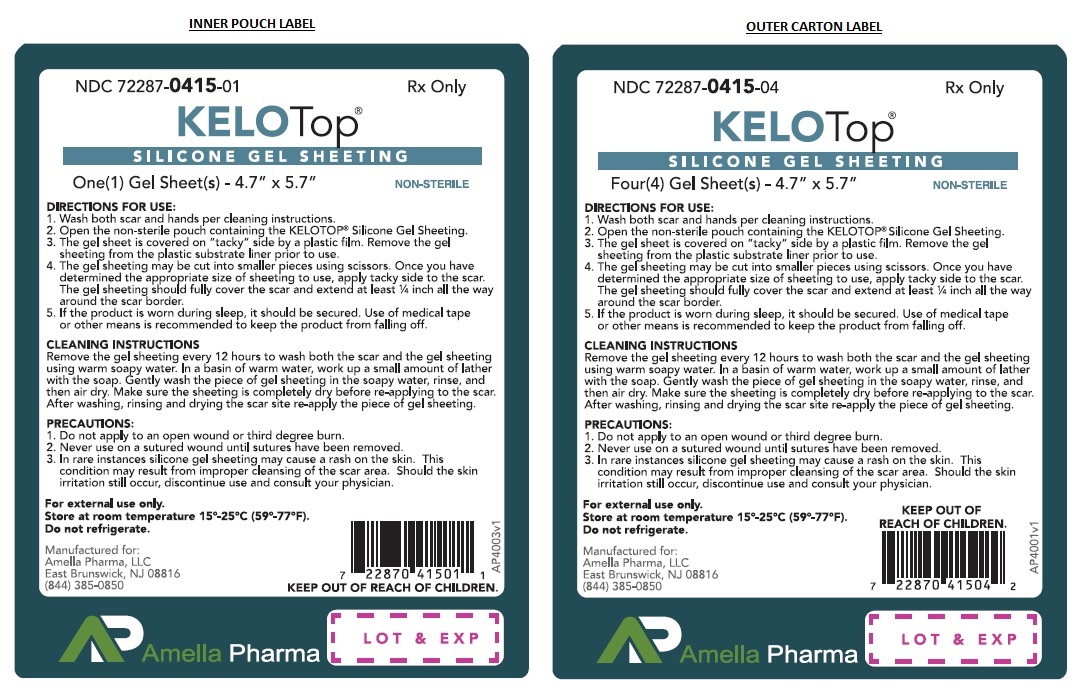
Label: KELOTOP - DIMETHYL METHYLHYDROGEN METHYLVINYL SILICONE GEL, ORGANOPOLYSILOXANE, SILICONE GEL SHEETING-
- NHRIC Code(s): 72287-415-04, 72287-415-01
- Packager: AMELLA PHARMA, LLC
- Category: MEDICAL DEVICE
- DEA Schedule: None
- Marketing Status: Premarket Notification
Drug Label Information
Updated December 26, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- KELOTOP® - DIMETHYL METHYLHYDROGEN METHYLVINYL SILICONE GEL, ORGANOPOLYSILOXANE, SILICONE GEL SHEETING
- INDICATIONS
- CONTRAINDICATIONS
-
WARNINGS, PRECAUTIONS, ADVERSE REACTIONS
Possible complications include:
• Superficial maceration of the skin
• Rash
• Skin Discoloration
• Pruritus
• Sheeting tack and thickness varyRashes have been observed on skin under the KELOTOP®, this has been attributed to poor or insufficient hygiene. Similar rashes have been attributed to KELOTOP® being wrapped too tightly. Should a rash occur, stop using the KELOTOP® for 12 hours followed by using the KELOTOP® for 12 hours. If the rash persists, a physician should be contacted and gel sheeting use should be discontinued.
Discoloration of the skin covered by KELOTOP® has been reported, particularly in dark skinned patients. This effect appears to be transient, and may be similar to the discoloration experienced whenever an area of skin is covered for extended periods of time. If ingested, get medical help or contact Poison Control Center right away.
Some patients report differences in KELOTOP® surface tack and thickness from sheet to sheet. Perception of tack is subjective and the adhesive characteristic of KELOTOP® may vary. These variations do not affect the function of the product.
Small bubbles may form on the KELOTOP® after repeated washing and use. This does not have an impact on the function of the product. This medication should be used as directed by your physician during pregnancy or while breastfeeding. Consult your doctor about the risks and benefits.
Do not use creams, lotions, sun block or other silicone products on your skin when wearing KELOTOP®. These products will create a barrier between the scar site and the silicone gel, preventing a proper healing environment.
Call your doctor about side effects. You may report side effects to FDA at 1-800-FDA-1088. KEEP OUT OF REACH OF CHILDREN.
PRECAUTIONS:
- Do not apply to an open wound or third degree burn.
- Never use on a sutured wound until sutures have been removed.
- In rare instances silicone gel sheeting may cause a rash on the skin. This condition may result from improper cleansing of the scar area. Should the skin irritation still occur, discontinue use and consult your physician
- INGREDIENTS
-
INSTRUCTIONS FOR USE:
- Wash both scar and hands per cleaning instructions.
- Open the non-sterile pouch containing the KELOTOP®.
- The gel sheet is covered on "tacky" side by a plastic film. Remove the KELOTOP® from the plastic substrate liner prior to use.
- The gel sheet may be cut into smaller pieces using scissors. Once you have determined the appropriate size of sheeting to use, apply tacky side to the scar. The gel sheeting should fully cover the scar and extend atleast ¼ inch all the way around the scar border.
- If the product is worn during sleep, it should be secured. Use of medical tape or other means is recommended to keep the product from falling off.
CLEANING INSTRUCTIONS Remove the gel sheeting every 12 hours to wash both the scar and the KELOTOP®. In a basin of warm water, work up a small amount of lather with the soap. Gently wash the piece of gel sheeting in the soapy water, rinse, and then air dry. Make sure the sheeting is completely dry before re-applying to the scar. After washing, rinsing and drying the scar site re-apply the piece of gel sheeting.
WEARING TIME Optimal wearing time for KELOTOP® is 24 hours per day. If it is not possible to wear the gel sheet for the recommended 24 hour period, a minimum of 12 hours per day is required, washing per the instructions above once in that period. Follow this procedure each day, washing and re-applying the gel sheeting to the scar for 7 days. At that time the piece of gel sheeting will begin to lose its adhesive quality and/or may become embedded with surface dirt. It is recommended to discard the piece of gel sheeting and apply a new piece.
The overall optimal period of use is usually 8 to 12 weeks.
-
HOW IT IS SUPPLIED
KELOTOP® are supplied as a clear gel matrix sheet sized 4.7” x 5.7” with a pink mylar cover to protect the sticky side, and a thick white translucent reusable mylar cover to protect the KELOTOP® between uses. Non-sterile product is labeled as such and supplied in a protective package within a protective outer container.
KELOTOP® is available as the following:
NDC 72287-415-04 4ct envelopeStore at 15°-25°C (59° to 77°F); Keep away from heat and protect from freezing. Do not refrigerate.
NON-STERILE
Manufactured for:
Amella Pharma, LLC
East Brunswick, NJ 0881612/2018 AP-4002v2
KELOTOP® is a registered Trademark of Amella Pharma, LLC - Packaging
-
INGREDIENTS AND APPEARANCE
KELOTOP - DIMETHYL METHYLHYDROGEN METHYLVINYL SILICONE GEL, ORGANOPOLYSILOXANE, SILICONE GEL SHEETING
elastomer, silicone, for scar managementProduct Information Product Type MEDICAL DEVICE Item Code (Source) NHRIC:72287-415 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:72287-415-04 4 in 1 PACKAGE 1 NHRIC:72287-415-01 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date premarket notification K003948 12/15/2018 Labeler - AMELLA PHARMA, LLC (081189492)