Label: HYDROCODONE BITARTRATE AND HOMATROPINE METHYLBROMIDE syrup
-
Contains inactivated NDC Code(s)
NDC Code(s): 54868-5168-0 - Packager: Physicians Total Care, Inc.
- This is a repackaged label.
- Source NDC Code(s): 50383-043
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIII
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 15, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
This product contains hydrocodone (dihydrocodeinone) bitartrate, a semisynthetic centrally-acting narcotic antitussive. Homatropine methylbromide is included in a subtherapeutic amount to discourage deliberate overdosage.
Each teaspoonful (5 mL) for oral administration contains:
Hydrocodone Bitartrate, USP 5 mg
WARNING: May be habit forming.
Homatropine Methylbromide, USP 1.5 mg
Hydrocodone Bitartrate and Homatropine Methylbromide Syrup also contains: Caramel color, cherry flavor, citric acid, FD&C Red #40, methylparaben, propylparaben, purified water, sorbitol solution and sucrose syrup. Citric acid and/or sodium citrate may be added to adjust pH.
The hydrocodone component is a 4,5α- epoxy-3-methoxy-17-methylmorphinan- 6-one tartrate (1:1) hydrate (2:5), a fine white crystal or crystalline powder, which is derived from the opium alkaloid, thebaine, has a molecular weight of (494.50) and may be represented by the following structural formula:
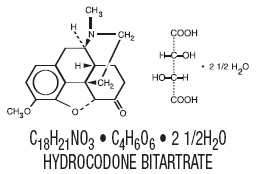
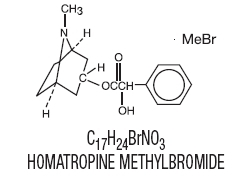
Homatropine methylbromide is 8- Azoniabicyclo [3.2.1]octane,3-[(hydroxyphenylacetyl) oxy]-8,8-dimethyl-,bromide, endo-; a white crystal or fine white crystalline powder, with a molecular weight of (370.29).
-
CLINICAL PHARMACOLOGY
Hydrocodone is a semisynthetic narcotic antitussive and analgesic with multiple actions qualitatively similar to those of codeine. The precise mechanism of action of hydrocodone and other opiates is not known; however, hydrocodone is believed to act directly on the cough center. In excessive doses, hydrocodone, like other opium derivatives, will depress respiration. The effects of hydrocodone in therapeutic doses on the cardiovascular system are insignificant. Hydrocodone can produce miosis, euphoria, physical and physiological dependence.
Following a 10 mg oral dose of hydrocodone administered to five adult male subjects, the mean peak concentration was 23.6 ± 5.2 ng/mL. Maximum serum levels were achieved at 1.3 ± 0.3 hours and the half-life was determined to be 3.8 ± 0.3 hours. Hydrocodone exhibits a complex pattern of metabolism including O-demethylation, N-demethylation and 6-keto reduction to the corresponding 6-α-and 6-β-hydroxymetabolites.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
May be habit forming. Hydrocodone can produce drug dependence of the morphine type and, therefore, has the potential for being abused. Psychic dependence, physical dependence and tolerance may develop upon repeated administration of hydrocodone bitartrate and homatropine methylbromide and it should be prescribed and administered with the same degree of caution appropriate to the use of other narcotic drugs (see DRUG ABUSE AND DEPENDENCE).
Respiratory Depression
Hydrocodone bitartrate and homatropine methylbromide produces dose-related respiratory depression by directly acting on brain stem respiratory centers. If respiratory depression occurs, it may be antagonized by the use of naloxone hydrochloride and other supportive measures when indicated.
Head Injury and Increased Intracranial Pressure
The respiratory depression properties of narcotics and their capacity to elevate cerebrospinal fluid pressure may be markedly exaggerated in the presence of head injury, other intracranial lesions or a pre-existing increase in intracranial pressure. Furthermore, narcotics produce adverse reactions which may obscure the clinical course of patients with head injuries.
Acute Abdominal Conditions
The administration of hydrocodone bitartrate and homatropine methylbromide or other narcotics may obscure the diagnosis or clinical course of patients with acute abdominal conditions.
Pediatric Use
In young pediatric patients, as well as adults, the respiratory center is sensitive to the depressant action of narcotic cough suppressants in a dose-dependent manner. Benefit to risk ratio should be carefully considered especially in the pediatric population with respiratory embarrassment (e.g., croup).
-
PRECAUTIONS
General
Before prescribing medication to suppress or modify cough, it is important to ascertain that the underlying cause of cough is identified, that modification of cough does not increase the risk of clinical or physiological complications, and that appropriate therapy for the primary disease is provided.
Special Risk Patients
Hydrocodone bitartrate and homatropine methylbromide should be given with caution to certain patients such as the elderly or debilitated, and those with severe impairment of hepatic or renal functions, hypothyroidism, Addison’s disease, prostatic hypertrophy or urethral stricture, asthma, and narrow-angle glaucoma.
Information for patients
Hydrocodone may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks such as driving a car or operating machinery. The patient using hydrocodone bitartrate and homatropine methylbromide should be cautioned accordingly.
Drug Interactions
Patients receiving narcotics, antihistamines, antipsychotics, antianxiety agents or other CNS depressants (including alcohol) concomitantly with hydrocodone bitartrate and homatropine methylbromide may exhibit an additive CNS depression. When combined therapy is contemplated, the dose of one or both agents should be reduced. The use of MAO inhibitors or tricyclic antidepressants with hydrocodone preparations may increase the effect of either the antidepressant or hydrocodone.
Carcinogenesis, mutagenesis, impairment of fertility
Studies of hydrocodone bitartrate and homatropine methylbromide in animals to evaluate the carcinogenic and mutagenic potential and the effect on fertility have not been conducted.
Pregnancy
Teratogenic Effects
Pregnancy Category C: Animal reproduction studies have not been conducted with hydrocodone bitartrate and homatropine methylbromide. It is also not known whether hydrocodone bitartrate and homatropine methylbromide can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Hydrocodone bitartrate and homatropine methylbromide should be given to a pregnant woman only if clearly needed.
Nonteratogenic effects
Babies born to mothers who have been taking opioids regularly prior to delivery will be physically dependent. The withdrawal signs include irritability and excessive crying, tremors, hyperactive reflexes, increased respiratory rate, increased stools, sneezing, yawning, vomiting and fever. The intensity of the syndrome does not always correlate with the duration of maternal opioid use or dose.
Labor and delivery
As with all narcotics, administration of hydrocodone bitartrate and homatropine methylbromide to the mother shortly before delivery may result in some degree of respiratory depression in the newborn, especially if higher doses are used.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from hydrocodone bitartrate and homatropine methylbromide, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
-
ADVERSE REACTIONS
To report SUSPECTED ADVERSE REACTIONS, contact Hi-Tech Pharmacal Co., Inc. at 1-800-262-9010 or FDA at 1-800-FDA- 1088 or www.fda.gov/medwatch.
Central Nervous System
Sedation, drowsiness, mental clouding, lethargy, impairment of mental and physical performance, anxiety, fear, dysphoria, dizziness, psychic dependence, mood changes.
Gastrointestinal System
Nausea and vomiting may occur; they are more frequent in ambulatory than in recumbent patients. Prolonged administration of hydrocodone bitartrate and homatropine methylbromide may produce constipation.
Genitourinary System
Ureteral spasm, spasm of vesicle sphincters and urinary retention have been reported with opiates.
Respiratory System
Hydrocodone bitartrate and homatropine methylbromide may produce dose-related respiratory depression by acting directly on brain stem respiratory centers (see OVERDOSAGE).
-
DRUG ABUSE AND DEPENDENCE
Hydrocodone bitartrate and homatropine methylbromide syrup is a Schedule III narcotic. Psychic dependence, physical dependence and tolerance may develop upon repeated administration of narcotics; therefore, hydrocodone bitartrate and homatropine methylbromide should be prescribed and administered with caution. However, psychic dependence is unlikely to develop when hydrocodone bitartrate and homatropine methylbromide is used for a short time for the treatment of cough. Physical dependence, the condition in which continued administration of the drug is required to prevent the appearance of a withdrawal syndrome, assumes clinically significant proportions only after several weeks of continued oral narcotic use, although some mild degree of physical dependence may develop after a few days of narcotic therapy.
-
OVERDOSAGE
Signs and Symptoms
Serious overdosage with hydrocodone is characterized by respiratory depression (a decrease in respiratory rate and/or tidal volume, Cheyne-Stokes respiration, cyanosis), extreme somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, and sometimes bradycardia and hypotension. In severe overdosage, apnea, circulatory collapse, cardiac arrest and death may occur. The ingestion of very large amounts of hydrocodone bitartrate and homatropine methylbromide may, in addition, result in acute homatropine intoxication.
Treatment
Primary attention should be given to the reestablishment of adequate respiratory exchange through provision of a patent airway and the institution of assisted or controlled ventilation. The narcotic antagonist naloxone hydrochloride is a specific antidote for respiratory depression which may result from overdosage or unusual sensitivity to narcotics including hydrocodone. Therefore, an appropriate dose of naloxone hydrochloride should be administered, preferably by the intravenous route, simultaneously with efforts at respiratory resuscitation. For further information, see full prescribing information for naloxone hydrochloride. An antagonist should not be administered in the absence of clinically significant respiratory depression. Oxygen, intravenous fluids, vasopressors and other supportive measures should be employed as indicated. Gastric emptying may be useful in removing unabsorbed drug.
- DOSAGE AND ADMINISTRATION
-
HOW SUPPLIED
Hydrocodone Bitartrate and Homatropine Methylbromide Syrup is available as a clear red colored, cherry flavored syrup in:
Bottles of 16 fl. oz. (one pint) ...... NDC 54868-5168-0
Store at 20°-25°C (68°-77°F) [See USP Controlled Room Temperature].
Dispense in a tight, light-resistant container, as defined in the USP.
Oral prescription where permitted by state law.
Manufactured By:
Hi-Tech Pharmacal Co., Inc.
Amityville, NY 11701
Rev. 043:00 3/09
MG #21271
Additional barcode label applied by:
Physicians Total Care, Inc.
Tulsa, Oklahoma 74146
-
PRINCIPAL DISPLAY PANEL

NDC 54868-5168-0
HYDROCODONE BITARTRATE AND HOMATROPINE METHYLBROMIDE SYRUP
5 mg/1.5 mg per 5 mL
Each 5 mL (teaspoonful) contains:
Hydrocodone Bitartrate, USP....................................5 mg
Homatropine Methylbromide, USP..........................1.5 mg
Usual Dosage: Read accompanying product information.
Dispense in a tight, light-resistant container as defined in the USP.
KEEPT TIGHTLY CLOSED.
Store at controlled room temperature 20° to 25°C (68° to 77°F) [see USP].
Oral prescription where permitted by state law.
Rx only
16 fl oz (473 mL)
-
INGREDIENTS AND APPEARANCE
HYDROCODONE BITARTRATE AND HOMATROPINE METHYLBROMIDE
hydrocodone bitartrate and homatropine methylbromide syrupProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54868-5168(NDC:50383-043) Route of Administration ORAL DEA Schedule CIII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCODONE BITARTRATE (UNII: NO70W886KK) (HYDROCODONE - UNII:6YKS4Y3WQ7) HYDROCODONE BITARTRATE 5 mg in 5 mL HOMATROPINE METHYLBROMIDE (UNII: 68JRS2HC1C) (HOMATROPINE - UNII:8QS6WCL55Z) HOMATROPINE METHYLBROMIDE 1.5 mg in 5 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) CARAMEL (UNII: T9D99G2B1R) CHERRY (UNII: BUC5I9595W) FD&C RED NO. 40 (UNII: WZB9127XOA) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) SODIUM CITRATE (UNII: 1Q73Q2JULR) SORBITOL (UNII: 506T60A25R) SUCROSE (UNII: C151H8M554) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54868-5168-0 1 in 1 CARTON 1 473 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040613 11/07/2007 Labeler - Physicians Total Care, Inc. (194123980) Establishment Name Address ID/FEI Business Operations Physicians Total Care, Inc. 194123980 relabel

