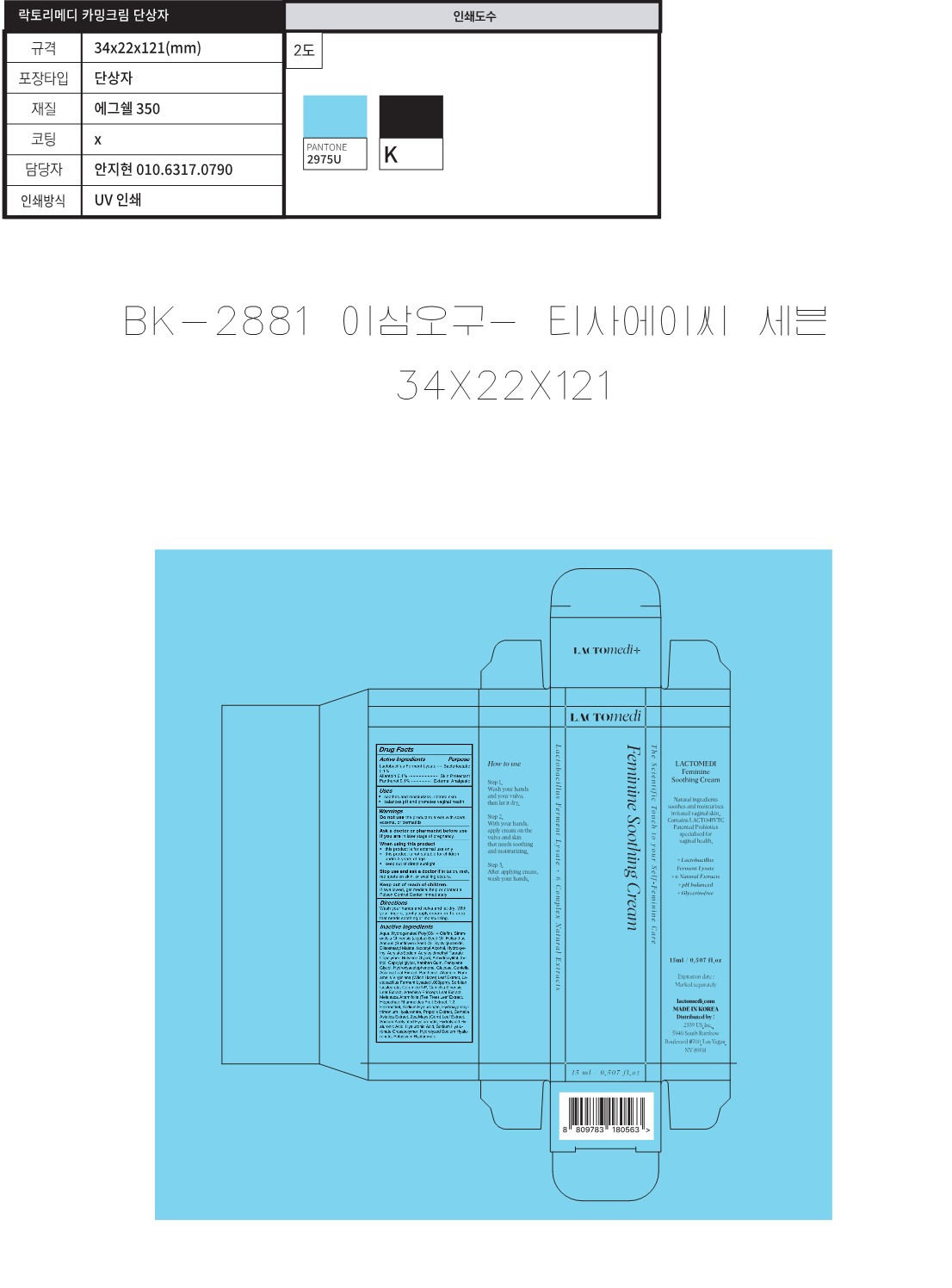
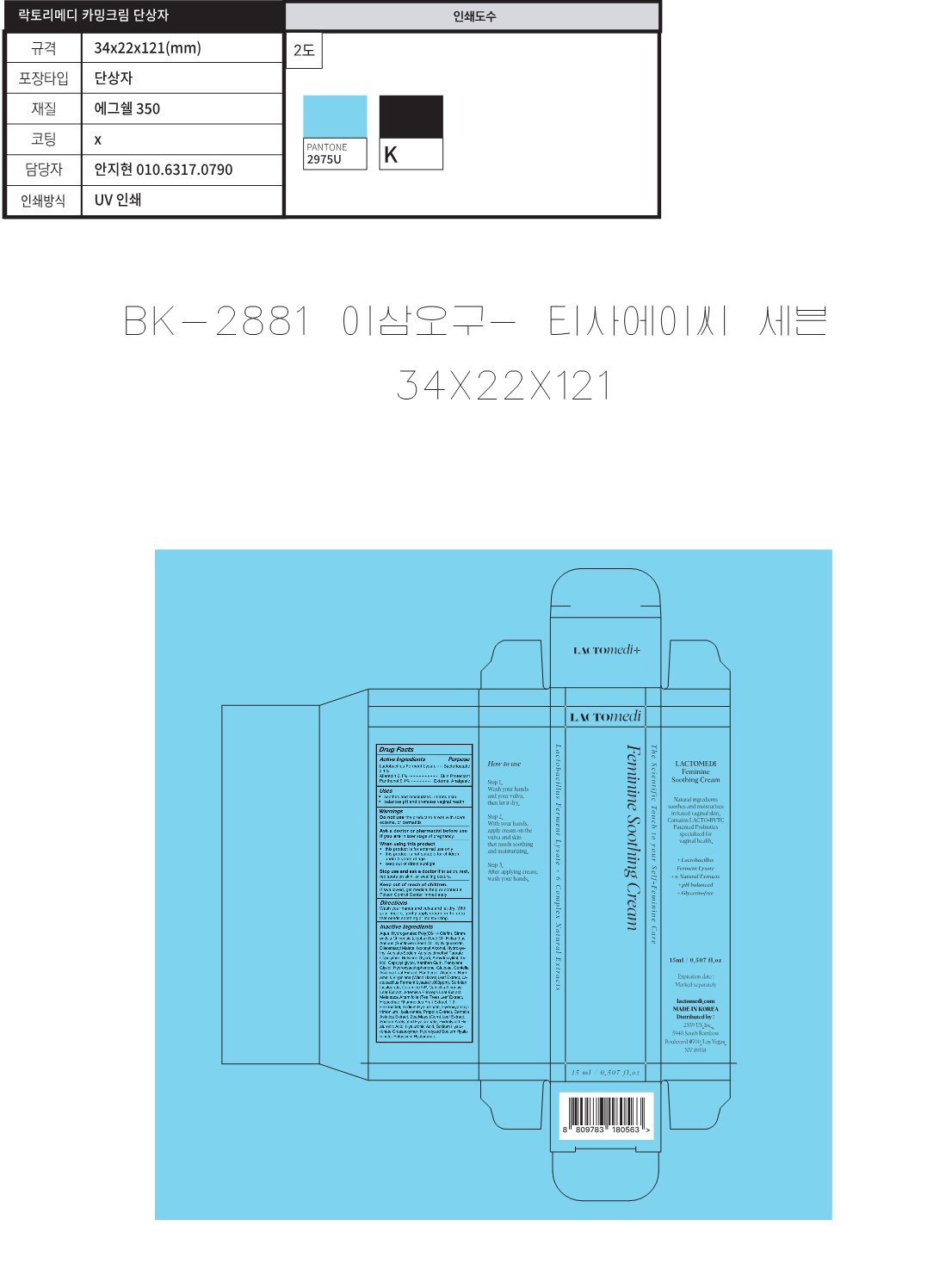
Label: LACTOMEDI FERMININE SOOTHING CREAM- lactobacillus ferment lysate, allantoin, panthenol cream
- NDC Code(s): 83490-317-01
- Packager: 2359 US INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 5, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purposes
- Uses
- Warnings
- Warnings
- Warnings
- Warnings
-
Warnings
Do not use the product to areas with scars, eczema, or dermatitis
Ask a doctor before use if you have
■ irritation
■ rash
■ red spots on skin
■ swellingAsk a doctor or pharmacist before use if you are in later stage of pregnancy.
When using this product
■ this product is for external use only
■ this product is not suitable for children under 3 years of age
■ keep out of direct sunlightKeep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center immediately. - Directions
-
Inactive Ingredients
Aqua, Hydrogenated Poly(C6-14 Olefin), Simmondsia Chinensis (Jojoba) Seed Oil,
Helianthus Annuus (Sunflower) Seed Oil, Xylitylglucoside, Diisostearyl Malate, Isocetyl Alcohol,
Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Butylene Glycol,
Anhydroxylitol, Xylitol, Caprylyl glycol, Xanthan Gum, Pentylene Glycol, Hydroxyacetophenone,
Glucose, Centella Asiatica Leaf Extract, Panthenol, Allantoin, Hamamelis Virginians (Witch Hazel) Leaf Extract,
Lactobacillus Ferment Lysate(1 .OOOpprn), Sorbit Isostearate, Ceramide NP, Camellia Sinensis Leaf Extract,
Artemisia Princeps Leaf Extract, Melaleuca Alternifolia (Tea Tree) Leaf Extract, Hippophae Rhamnoides Fruit Extract,
1,2-Hexanediol, Sodium Hyaluronate, Hydroxypropyl trimonium Hyaluronate, Propolis Extract,
Centell Asiatica Extract, Zea Mays (Corn) Leaf Extract, Sodium Acetylated Hyaluronate, Hydrolyzed Hyaluronic Acid,
Hyaluronic Acid, Sodium Hyaluronate Crosspolymer, Hydrolyzed Sodium Hyaluronate, Potassium Hyaluronate
- Label
-
INGREDIENTS AND APPEARANCE
LACTOMEDI FERMININE SOOTHING CREAM
lactobacillus ferment lysate, allantoin, panthenol creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83490-317 Route of Administration VAGINAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIMOSILACTOBACILLUS FERMENTUM (UNII: 2C1F12C6AP) (LIMOSILACTOBACILLUS FERMENTUM - UNII:2C1F12C6AP) LIMOSILACTOBACILLUS FERMENTUM 1 g in 100 mL PANTHENOL (UNII: WV9CM0O67Z) (PANTHENOL - UNII:WV9CM0O67Z) PANTHENOL 1 g in 100 mL ALLANTOIN (UNII: 344S277G0Z) (ALLANTOIN - UNII:344S277G0Z) ALLANTOIN 1 g in 100 mL Inactive Ingredients Ingredient Name Strength ANHYDROXYLITOL (UNII: 8XWR7NN42F) HYALURONIC ACID (UNII: S270N0TRQY) HYALURONATE SODIUM (UNII: YSE9PPT4TH) POTASSIUM (UNII: RWP5GA015D) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (HARD PARTICLE) (UNII: H895X08VNQ) VINYL DIMETHICONE/METHICONE SILSESQUIOXANE CROSSPOLYMER (UNII: 9NH1UDD2RR) HYDROLYSED MARINE COLLAGEN (ENZYMATIC; 2000 MW) (UNII: 2WID9OCG7P) ETHYL OLIVATE (UNII: KKJ108Y20W) PEG-9 DIGLYCIDYL ETHER/SODIUM HYALURONATE CROSSPOLYMER (UNII: 788QAG3W8A) HYDROGENATED POLYDECENE TYPE I (UNII: U333RI6EB7) FIBROBLAST GROWTH FACTOR-1 (UNII: G53298VN9Y) ALANINE (UNII: OF5P57N2ZX) WATER (UNII: 059QF0KO0R) PROPOLIS WAX (UNII: 6Y8XYV2NOF) HAMAMELIS VIRGINIANA LEAF (UNII: T07U1161SV) GREEN TEA LEAF (UNII: W2ZU1RY8B0) JOJOBA OIL (UNII: 724GKU717M) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) ISOCETYL ALCOHOL (UNII: 1800H64066) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (100000 MPA.S AT 1.5%) (UNII: 86FQE96TZ4) XANTHAN GUM (UNII: TTV12P4NEE) PENTYLENE GLYCOL (UNII: 50C1307PZG) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) SUNFLOWER OIL (UNII: 3W1JG795YI) CENTELLA ASIATICA LEAF (UNII: 6810070TYD) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) ARTEMISIA PRINCEPS LEAF (UNII: SY077EW02G) XYLITYLGLUCOSIDE (UNII: O0IEZ166FB) CERAMIDE NP (UNII: 4370DF050B) HIPPOPHAE RHAMNOIDES FRUIT (UNII: AVL0R9111T) HYDROGENATED POLY(C6-14 OLEFIN; 2 CST) (UNII: P0TX083987) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) CORN LEAF (UNII: LF3J87254C) SODIUM ACETYLATED HYALURONATE (UNII: WN66R7GL93) CENTELLA ASIATICA TRITERPENOIDS (UNII: 4YS74Q4G4J) XYLITOL (UNII: VCQ006KQ1E) CAPRYLYL GLYCOL (UNII: 00YIU5438U) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83490-317-01 15 mL in 1 TUBE; Type 0: Not a Combination Product 08/22/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M016 08/22/2023 Labeler - 2359 US INC (118974080) Registrant - 2359 US INC (118974080) Establishment Name Address ID/FEI Business Operations Isamogu Inc. 695695834 manufacture(83490-317)