Label: OMEGA3 FISH OIL capsule, gelatin coated
- NHRIC Code(s): 25685-100-01
- Packager: Regimed Medical
- Category: DIETARY SUPPLEMENT
- DEA Schedule: None
- Marketing Status: Dietary Supplement
Drug Label Information
Updated December 16, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
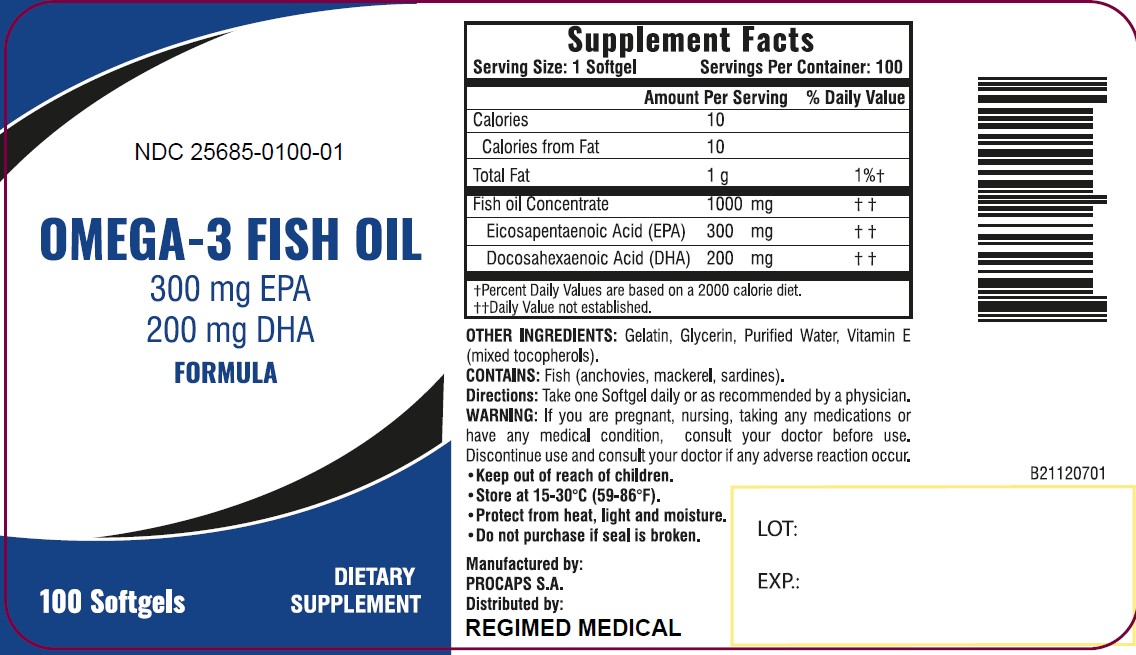
PRINCIPAL DISPLAY PANEL
NDC 25685-0100-01
OMEGA-3 FISH OIL
300 mg EPA
200 mg DHA
FORMULA
100 Softgels
DIETARY SUPPLEMENT
Supplement Facts Serving Size: 1 Softgel Servings Per Container: 100 Amount Per Serving % Daily Value Calories 10 Calories from Fat 10 Total Fat 1 g 1% † Fish oil Concentrate 1000 mg † † Elcosapentaenoic Acid (EPA) 300 mg † † Docosahexaenoic Acid (DHA) 200 mg † † † Percent Daily Values are based on a 2000 calorie diet. †† Daily Value not established. OTHER INGREDIENTS: Gelatin, Glycerin, Purified Water, Vitamin E (mixed tocopherols)
CONTAINS: Fish (anchovies, mackerel, sardines).
Directions: Take one Softgel daily or as recommended by a physician.
WARNING: If you are pregnant, nursing, taking any medications or have any medical condition, consult your doctor before use. Discontinue use and consult your doctor if any adverse reaction occur.
- Keep out of reach of children.
- Store at 15-30°C (59-86°F).
- Protect from heat, light and moisture.
- Do not purchase if seal is broken.
Manufactured by:
PROCAPS S.A.
Distributed by:
REGIMED MEDICAL
- STATEMENT OF IDENTITY
-
INGREDIENTS AND APPEARANCE
OMEGA3 FISH OIL
omega3 fish oil capsule, gelatin coatedProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:25685-100 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FISH OIL (UNII: XGF7L72M0F) (FISH OIL - UNII:XGF7L72M0F) FISH OIL 1000 mg Inactive Ingredients Ingredient Name Strength GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) TOCOPHEROL (UNII: R0ZB2556P8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:25685-100-01 100 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date dietary supplement 12/16/2021 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color shape size (solid drugs) 23 mm scoring 1 Labeler - Regimed Medical (809916570) Registrant - Regimed Medical (809916570) Establishment Name Address ID/FEI Business Operations Procaps S.A. 883237850 manufacture(25685-100)