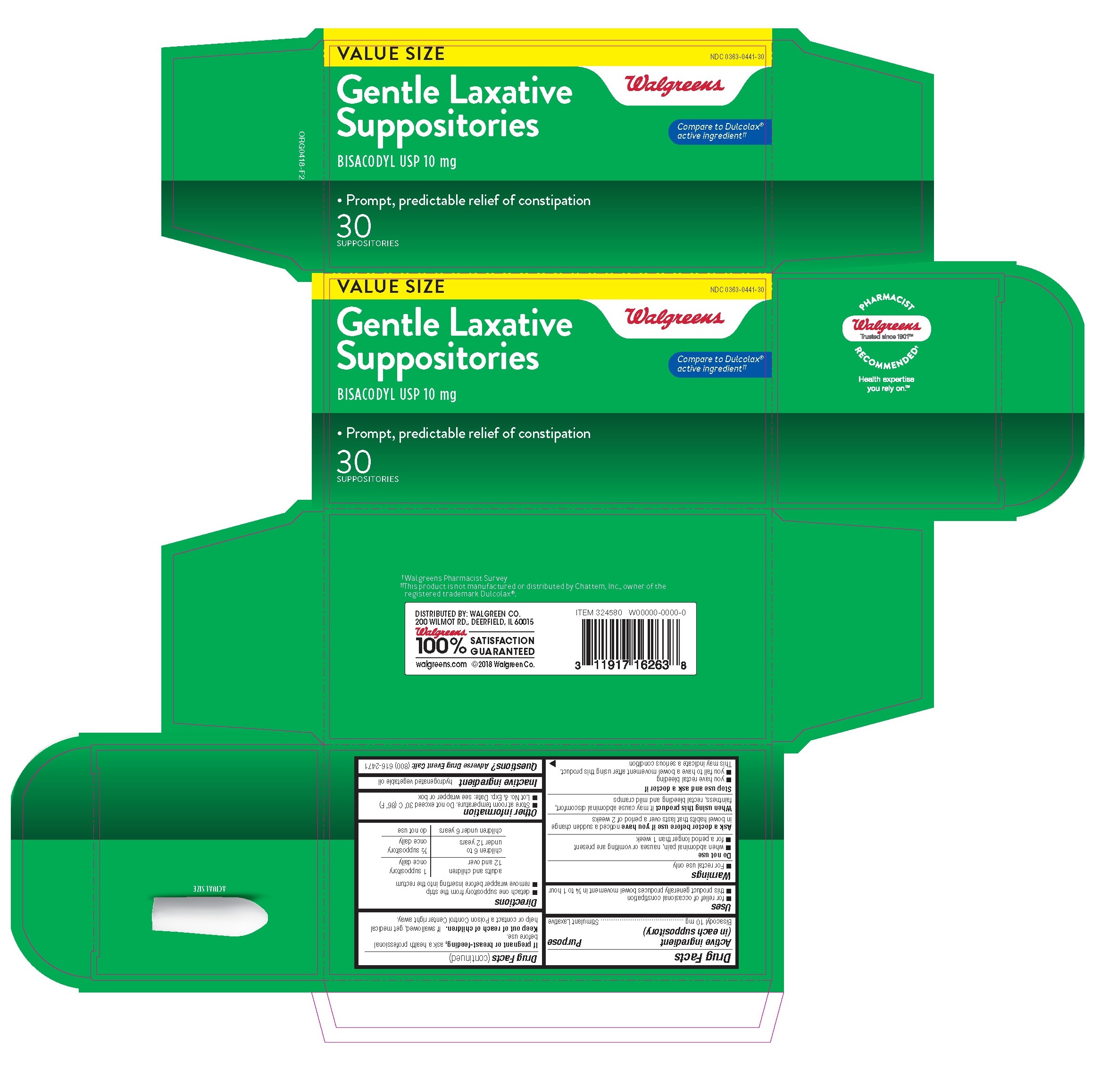
Label: WALGREENS GENTLE LAXATIVE- bisacodyl suppository suppository
- NDC Code(s): 0363-0466-30
- Packager: Walgreens
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 24, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient (in each Suppository)
- Uses
- Warnings
- Do not use
- Ask a doctor
- When using this product
- Stop use and ask a doctor if
- If pregnant or breast-feeding
- Keep out of reach of children
- Directions
- Other information
- Inactive ingredient
- Directions
- Label
-
INGREDIENTS AND APPEARANCE
WALGREENS GENTLE LAXATIVE
bisacodyl suppository suppositoryProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0363-0466 Route of Administration RECTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BISACODYL (UNII: 10X0709Y6I) (DEACETYLBISACODYL - UNII:R09078E41Y) BISACODYL 10 mg Inactive Ingredients Ingredient Name Strength PALM KERNEL OIL (UNII: B0S90M0233) Product Characteristics Color white Score Shape BULLET Size 32mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0363-0466-30 5 in 1 PACKAGE 09/18/2014 1 6 in 1 CARTRIDGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M007 09/18/2014 Labeler - Walgreens (008965063) Registrant - Unipack LLC (116015769) Establishment Name Address ID/FEI Business Operations Unipack LLC 009248480 manufacture(0363-0466)