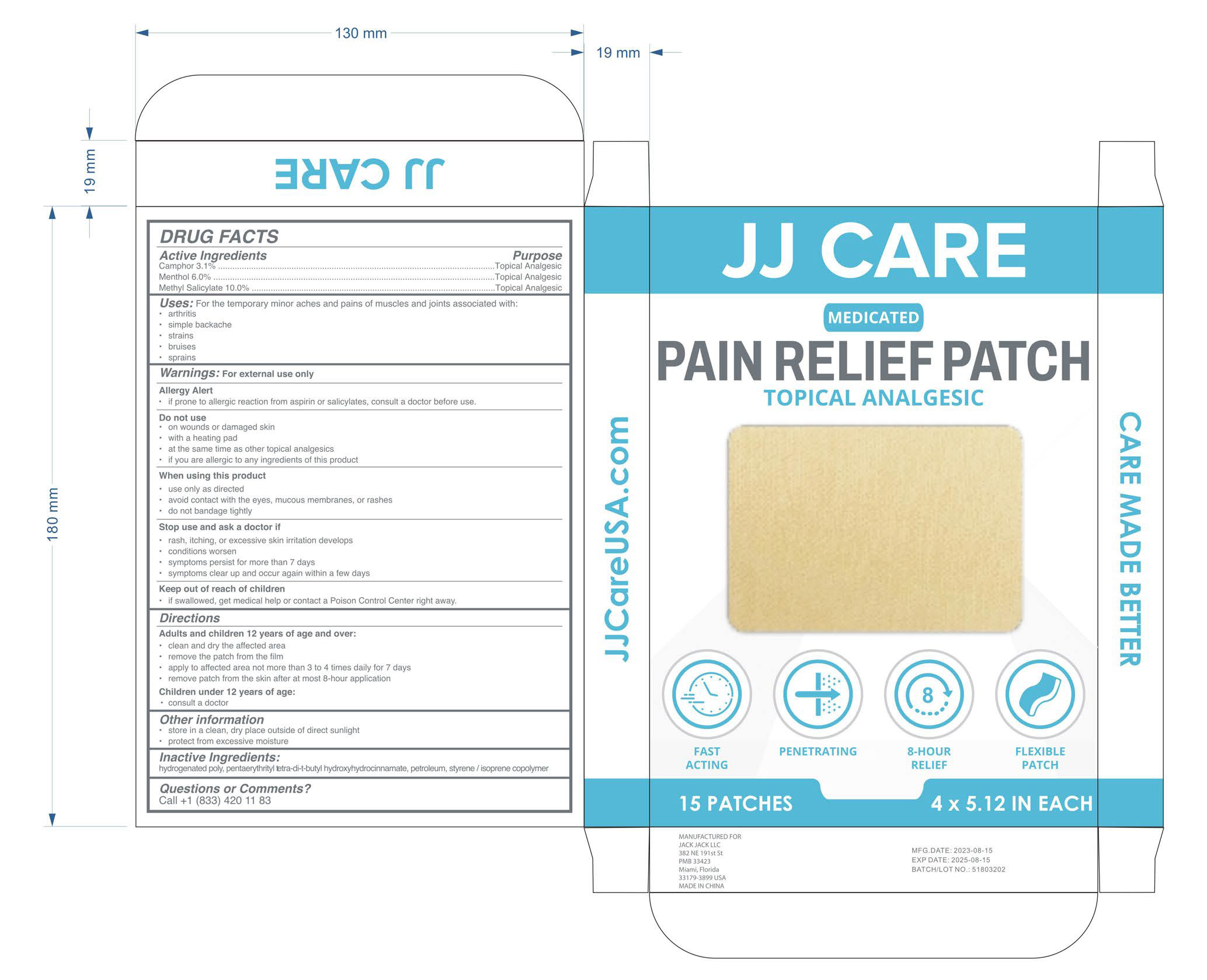
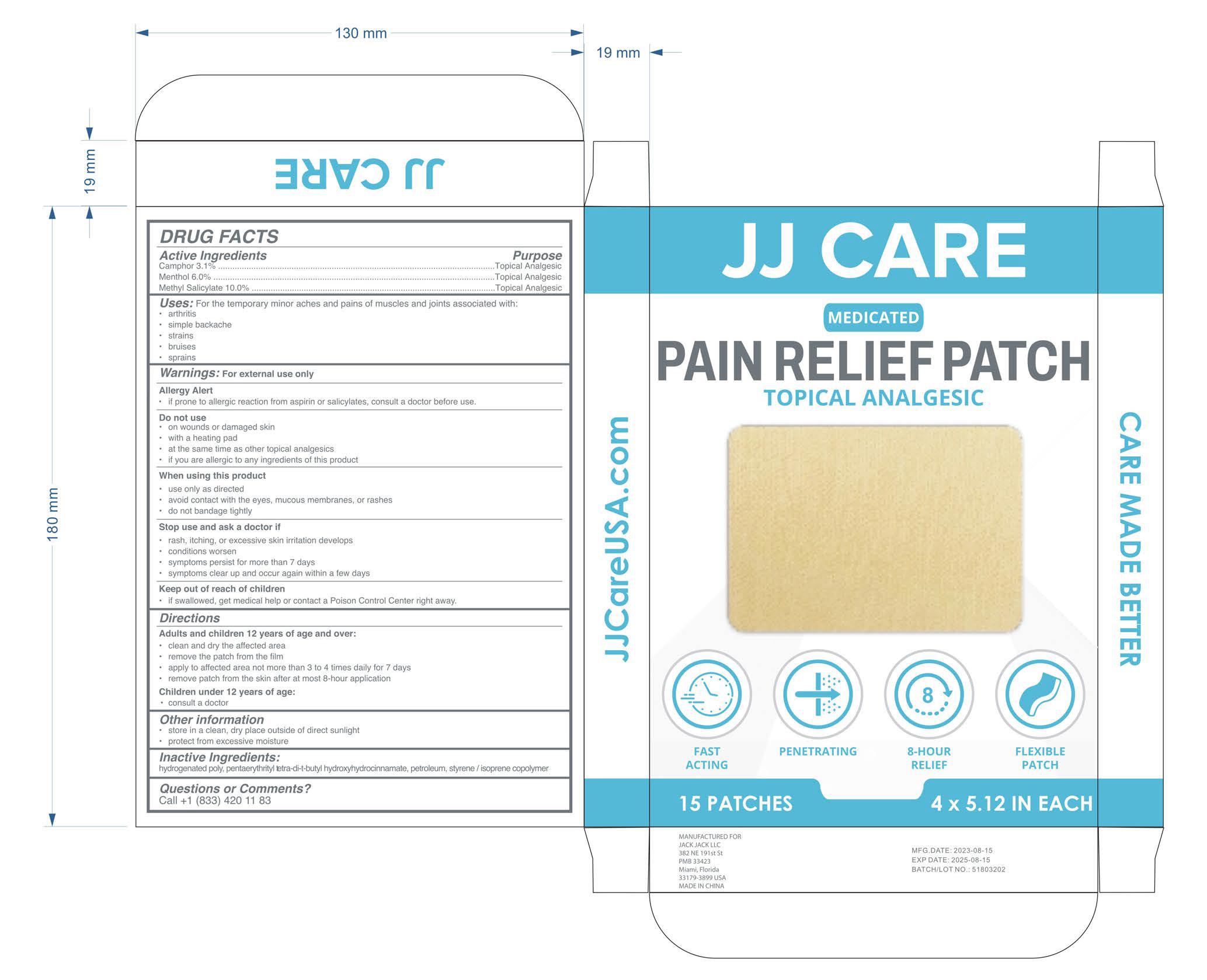
Label: PAIN RELIEF PATCH- camphor, menthol, methyl salicylate patch
- NDC Code(s): 81484-801-01, 81484-801-02
- Packager: Anhui Miao De Tang Pharmaceutical Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 14, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PAIN RELIEF PATCH
camphor, menthol, methyl salicylate patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:81484-801 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 3.1 mg in 100 METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 10 mg in 100 MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 6 mg in 100 Inactive Ingredients Ingredient Name Strength HYDROGENATED POLYISOBUTENE 8 (UNII: 7YR4ZFS62E) NAPHTHA (UNII: O3L624621X) ETHYL P-HYDROXYHYDROCINNAMATE (UNII: 8R568DFF4T) STYRENE (UNII: 44LJ2U959V) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81484-801-02 15 in 1 BOX 07/26/2023 1 NDC:81484-801-01 1 in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 07/26/2023 Labeler - Anhui Miao De Tang Pharmaceutical Co., Ltd. (405744102) Registrant - Anhui Miao De Tang Pharmaceutical Co., Ltd. (405744102) Establishment Name Address ID/FEI Business Operations Anhui Miao De Tang Pharmaceutical Co., Ltd. 405744102 manufacture(81484-801)