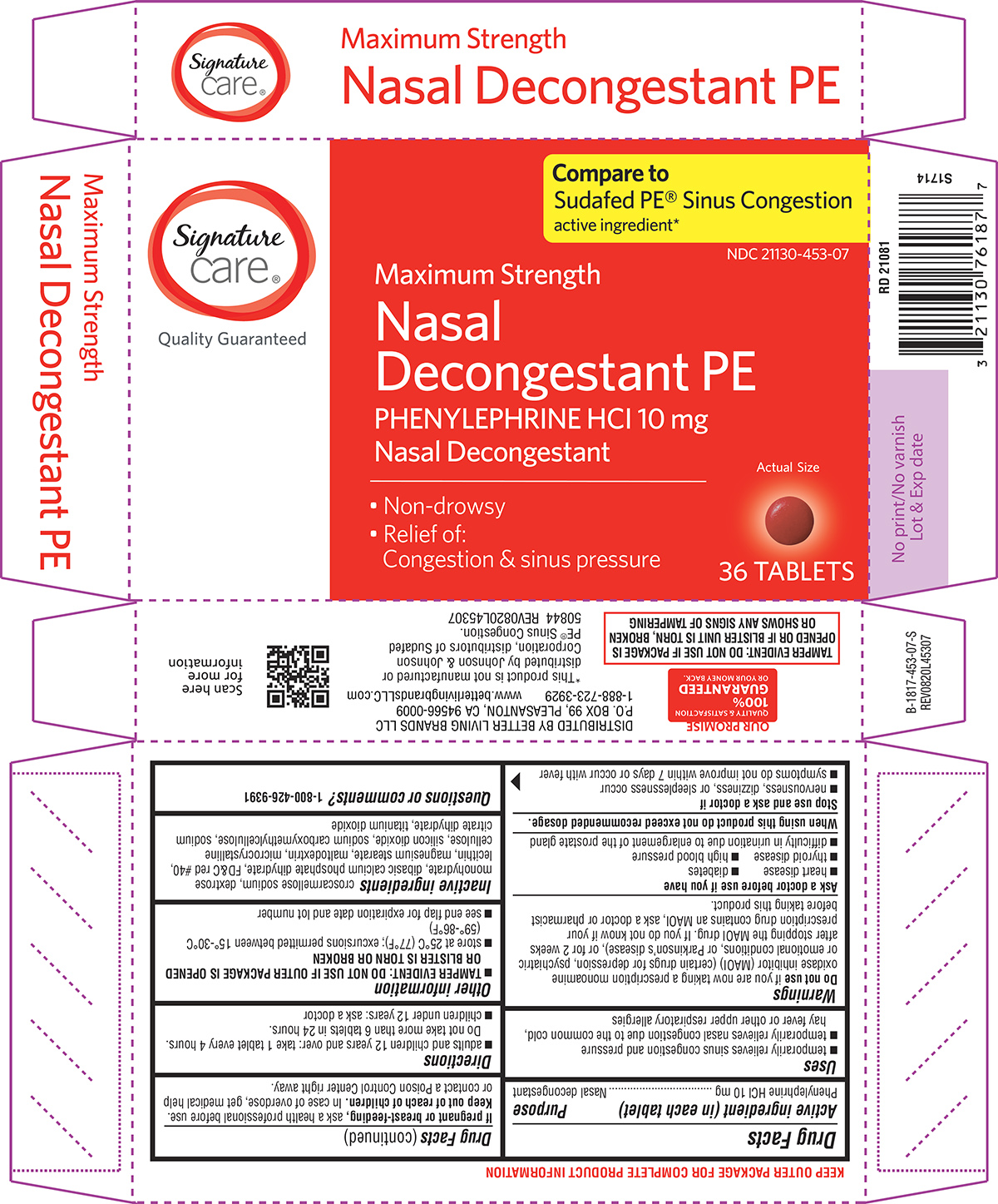
Label: NASAL DECONGESTANT PE- phenylephrine hcl tablet, film coated
- NDC Code(s): 21130-453-07, 21130-453-44
- Packager: Better Living Brands, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated August 16, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Do not use
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- heart disease
- diabetes
- thyroid disease
- high blood pressure
- difficulty in urination due to enlargement of the prostate gland
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
Signature
care®
Quality GuaranteedCompare to
Sudafed PE® Sinus Congestion
active ingredient*NDC 21130-453-07
Maximum Strength
Nasal
Decongestant PE
PHENYLEPHRINE HCI 10 mg
Nasal Decongestant• Non-drowsy
• Relief of:
Congestion & sinus pressureActual Size
36 TABLETS
TAMPER EVIDENT: DO NOT USE IF PACKAGE IS
OPENED OR IF BLISTER UNIT IS TORN, BROKEN
OR SHOWS ANY SIGNS OF TAMPERING*This product is not manufactured or
distributed by Johnson & Johnson
Corporation, distributors of Sudafed
PE® Sinus Congestion.
50844 REV0820L45307DISTRIBUTED BY BETTER LIVING BRANDS LLC
P.O. BOX 99, PLEASANTON, CA 94566-0009
1-888-723-3929 www.betterlivingbrandsLLC.com
OUR PROMISE
QUALITY & SATISFACTION
100%
GUARANTEED
OR YOUR MONEY BACK.
Signature Care 44-453
-
INGREDIENTS AND APPEARANCE
NASAL DECONGESTANT PE
phenylephrine hcl tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:21130-453 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 10 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) DEXTROSE MONOHYDRATE (UNII: LX22YL083G) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) FD&C RED NO. 40 (UNII: WZB9127XOA) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color red Score no score Shape ROUND Size 7mm Flavor Imprint Code 44;453 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:21130-453-07 2 in 1 CARTON 01/14/2005 1 18 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:21130-453-44 1 in 1 CARTON 01/14/2005 12/15/2017 2 18 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 01/14/2005 Labeler - Better Living Brands, LLC (009137209) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 manufacture(21130-453) , pack(21130-453) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867894 manufacture(21130-453) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 868734088 manufacture(21130-453) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 117025878 manufacture(21130-453)