Label: TYRVAYA- varenicline spray
- NDC Code(s): 73521-030-02, 73521-030-90
- Packager: Oyster Point Pharma, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated February 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use TYRVAYA safely and effectively. See full prescribing information for TYRVAYA.
TYRVAYA® (varenicline solution) nasal spray
Initial U.S. Approval: 2006
INDICATIONS AND USAGE
TYRVAYA (varenicline solution) nasal spray is a cholinergic agonist indicated for the treatment of the signs and symptoms of dry eye disease. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Nasal spray delivering 0.03 mg of varenicline in each spray (0.05 mL). (3)
CONTRAINDICATIONS
None. (4)
ADVERSE REACTIONS
The most common adverse reaction reported in 82% of patients was sneezing. Events that were reported in 5-16% of patients were cough, throat irritation, and instillation-site (nose) irritation. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Oyster Point Pharma at 1-877-EYE-0123 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 2/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosing Information
2.2 Priming Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
- 2 DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In three clinical studies of dry eye disease conducted with varenicline solution nasal spray, 349 patients received at least 1 dose of TYRVAYA. The majority of patients had 31 days of treatment exposure, with a maximum exposure of 105 days.
The most common adverse reactions reported in 82% of TYRVAYA treated patients was sneezing. Other common adverse reactions that were reported in >5% of patients include cough (16%), throat irritation (13%), and instillation-site (nose) irritation (8%).
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on TYRVAYA use in pregnant women to inform any drug associated risks. In animal reproduction studies, varenicline did not produce malformations at clinically relevant doses.
All pregnancies have a risk of birth defect, loss, or other adverse outcomes. In the US general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
Pregnant rats and rabbits received varenicline succinate during organogenesis at oral doses up to 15 and 30 mg/kg/day, respectively. While no fetal structural abnormalities occurred in either species, maternal toxicity, characterized by reduced body weight gain, and reduced fetal weights occurred in rabbits at the highest dose (4864 times the MRHD on a mg/m2 basis).
In a pre- and postnatal development study, pregnant rats received up to 15 mg/kg/day of oral varenicline succinate from organogenesis through lactation. Maternal toxicity, characterized by a decrease in body weight gain, was observed at 15 mg/kg/day (1216 times the MRHD on a mg/m2 basis). Decreased fertility and increased auditory startle response occurred in offspring at the highest maternal dose of 15 mg/kg/day.
8.2 Lactation
Risk Summary
There are no data on the presence of varenicline in human milk, the effects on the breastfed infant, or the effects on milk production. In animal studies varenicline was present in milk of lactating rats. However, due to species-specific differences in lactation physiology, animal data may not reliably predict drug levels in human milk.
The lack of clinical data during lactation precludes a clear determination of the risk of TYRVAYA to an infant during lactation; however, the developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for TYRVAYA and any potential adverse effects on the breastfed child from TYRVAYA.
-
11 DESCRIPTION
TYRVAYA nasal spray contains varenicline which is a partial nicotinic acetylcholine receptor agonist of α4β2, α4α6β2, α3β4, and α3α5β4 receptors and a full α7 receptor agonist.
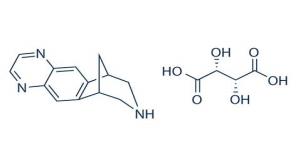
Varenicline, as the tartrate salt, is a powder which is a white to off-white to slightly yellow solid whose chemical name is 7,8,9,10-tetrahydro-6,10-methano-6H-pyrazino[2,3-h][3]benzazepine, (2R,3R)-2,3-dihydroxybutanedioate (1:1). It is highly soluble in water. Varenicline tartrate has a molecular weight of 361.35 Daltons and a molecular formula of C13H13N3 ⋅ C4H6O6. The chemical structure is:
TYRVAYA (varenicline solution) nasal spray is formulated for intranasal use as a clear 0.6 mg/mL strength solution, at pH 6.4. After priming [see Dosage and Administration (2.2)], each actuation delivers a 0.05 mL spray containing 0.03 mg varenicline free base, equivalent to 0.05 mg of varenicline tartrate. The formulation also contains the following inactive ingredients: sodium phosphate dibasic heptahydrate, monobasic sodium phosphate anhydrous, sodium chloride, sodium hydroxide and/or hydrochloric acid (to adjust pH) and water for injection.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The efficacy of TYRVAYA in dry eye disease is believed to be the result of varenicline's activity at heteromeric sub-type(s) of the nicotinic acetylcholine (nACh) receptor where its binding produces agonist activity and activates the trigeminal parasympathetic pathway resulting in increased production of basal tear film as a treatment for dry eye disease. Varenicline binds with high affinity and selectivity at human α4β2, α4α6β2, α3β4, α3α5β4 and α7 neuronal nicotinic acetylcholine receptors. The exact mechanism of action is unknown at this time.
12.3 Pharmacokinetics
Absorption/Distribution
Following administration of 0.12 mg (0.06 mg per 50-µL spray in each nostril), a strength of varenicline that is higher than the labeled concentration, varenicline can be detected in plasma by 5 minutes, generally achieves peak concentration within 2 hours, with a mean Cmax of 0.34 ng/mL, and has an AUC0-inf of 7.46 h*ng/mL. The systemic exposure (AUC0-inf) following this intranasal dose was approximately 7.5% of the exposure observed following a 1 mg oral dose of varenicline.
Metabolism/Elimination
The mean ± SD elimination half-life of varenicline after intranasal administration is approximately 19 ± 10 hours. Varenicline undergoes minimal metabolism with 92% excreted as unchanged drug in the urine.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Lifetime carcinogenicity studies were performed in CD-1 mice and Sprague-Dawley rats. There was no evidence of a carcinogenic effect in mice administered varenicline by oral gavage for 2 years at doses up to 20 mg/kg/day (810 times the maximum recommended human dose [MRHD], on a mg/m2 basis). Rats were administered varenicline (1, 5, and 15 mg/kg/day) by oral gavage for 2 years. In male rats (n = 65 per sex per dose group), incidences of hibernoma (tumor of the brown fat) were increased at the mid dose (1 tumor, 5 mg/kg/day, 405 times the MRHD on a mg/m2 basis) and maximum dose (2 tumors, 15 mg/kg/day, 1216 times the MRHD on a mg/m2 basis). The clinical relevance of this finding to humans has not been established. There was no evidence of carcinogenicity in female rats.
Mutagenesis
Varenicline was not genotoxic, with or without metabolic activation, in the following assays: Ames bacterial mutation assay; mammalian CHO/HGPRT assay; and tests for cytogenetic aberrations in vivo in rat bone marrow and in vitro in human lymphocytes.
Impairment of Fertility
There was no evidence of impairment of fertility in either male or female Sprague-Dawley rats administered varenicline succinate up to 15 mg/kg/day (1216 times the MRHD on a mg/m2 basis). Maternal toxicity, characterized by a decrease in body weight gain, was observed at 15 mg/kg/day. A decrease in fertility was noted in the offspring of pregnant rats administered varenicline succinate at an oral dose of 15 mg/kg/day. The decrease in fertility in the offspring of treated female rats was not evident at an oral dose of 3 mg/kg/day (243 times the MRHD, on a mg/m2 basis).
-
14 CLINICAL STUDIES
The efficacy of TYRVAYA for the treatment of dry eye disease was supported by two randomized, multi-center, double-masked, vehicle-controlled studies (ONSET-1 and ONSET-2). In the ONSET-1 study, 182 patients were randomized in a 1:1:1:1 ratio to receive one spray in each nostril twice daily of varenicline solution 0.006 mg (N=47), TYRVAYA 0.03 mg (N=48), varenicline solution 0.06 mg (N=44), or vehicle (N=43). In the ONSET-2 study, 758 patients were randomized in a 1:1:1 ratio to receive one spray in each nostril twice daily of TYRVAYA 0.03 mg (N=260), varenicline solution 0.06 mg (N=246), or vehicle (N=252).
The majority of patients were female (74%), the mean (standard deviation [SD]) age was 61 (12.5) years, the mean (SD) baseline anesthetized Schirmer’s score was 5.1 mm (2.9), and the mean (SD) baseline eye dryness score (EDS) was 59.3 (21.6). Use of artificial tears was allowed during the studies. Enrollment criteria included minimal signs [i.e., anesthetized Schirmer's test score (range, 0-10 mm) and corneal fluorescein staining (range, 2-14)] and was not limited by baseline EDS (range, 2-100).
Efficacy
Tear film production was measured by anesthetized Schirmer’s score assessed using a Schirmer’s strip (0-35 mm). The average baseline Schirmer’s score was 5.0 mm in the ONSET-1 study and 5.1 mm in the ONSET-2 study. Of the patients treated with TYRVAYA, 52% achieved ≥10 mm increase in Schirmer’s score from baseline in the ONSET-1 study and 47% achieved ≥10 mm increase in Schirmer’s score from baseline in the ONSET-2 study, compared to 14% and 28% of vehicle-treated patients in the ONSET-1 study and the ONSET-2 study, respectively at Day 28 (see Table 1). Of the patients treated with TYRVAYA, the mean change in Schirmer’s score was 11.7 mm and 11.3 mm as compared to 3.2 mm and 6.3 mm in the vehicle treated patients in the ONSET-1 study and ONSET-2 study, respectively at Day 28.
Table 1: Percent of Patients Achieving ≥10 mm Improvement from Baseline in Schirmer’s Score in 28-day Studies in Patients with Dry Eye Disease ONSET-1
ONSET-2
TYRVAYA
N=48Vehicle
N=43TYRVAYA
N=260Vehicle
N=252≥ 10-mm increase in tear production (% of eyes) at Day 28
52%
14%
47%
28%
Proportion Difference (95% CI)
38% (21%, 56%)
20% (11%, 28%)
p-value versus control
<0.01
<0.01
Cochran-Mantel-Haenszel (CMH) test controlling for study site, baseline Schirmer’s test score (STS), and baseline EDS.
All randomized and treated patients were included in the analysis and missing data were imputed using last-available data.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
TYRVAYA (varenicline solution) nasal spray is available in a carton containing two (2) nasal spray amber glass Type I bottles. Each bottle consists of a white nasal pump and blue dust cover, delivering 0.03 mg varenicline per spray (0.05 mL). Each bottle delivers one spray in each nostril twice daily for 15 days.
Two nasal spray bottles in each carton, containing 60 sprays per bottle, equivalent to 30-days’ supply with one spray in each nostril twice daily (NDC 73521-030-02).
-
17 PATIENT COUNSELING INFORMATION
- •
- Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
- •
- Instruct patients that TYRVAYA works to increase tear production in the eye after being sprayed in the nose.
- •
- Instruct patients to prime the bottle before using it for the first time by pumping seven (7) sprays into the air away from the face and to re-prime it by pumping 1 spray into the air away from the face if the bottle has not been used in more than five (5) days.
- •
- Instruct patients to wipe the nasal applicator with a clean tissue after each use.
- •
- Instruct patients to not shake or freeze the bottle.
Manufactured for: Oyster Point Pharma, Inc., a Viatris company, 202 Carnegie Center, Suite 106, Princeton, NJ 08540
Copyrights and Trademarks are property of their respective owners.
TYRVAYA® is a registered trademark of Oyster Point Pharma, Inc., a Viatris company.
TYRVAYA® and/or the use of TYRVAYA® in a method may be covered by one or more patents or patent applications, available at www.oysterpointrx.com/patent-notices.
©2023 Oyster Point Pharma, Inc., a Viatris Company. All Rights Reserved.
OYP:TYRVA:R1
-
Patient Package Insert
TYRVAYA® (Teer-vye-ah)
(varenicline solution)
nasal spray, for intranasal use
What is TYRVAYA?
TYRVAYA is a prescription nasal spray used to treat the signs and symptoms of dry eye disease.
Before you use TYRVAYA, tell your healthcare provider about all of your medical conditions, including if you:
- •
- are pregnant or plan to become pregnant. It is not known if TYRVAYA will harm your unborn baby.
- •
- are breastfeeding or plan to breastfeed. It is not known if TYRVAYA passes into your breast milk. You and your healthcare provider should decide if you will use TYRVAYA if you plan to breastfeed.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I use TYRVAYA?
- •
- See the Instructions for Use at the end of this Patient Information leaflet for information about the right way to use TYRVAYA.
- •
- TYRVAYA increases tear production in the eye after being sprayed in the nose.
- •
- Use TYRVAYA exactly as your healthcare provider tells you to use it.
- •
- Do not shake the bottles.
- •
- Spray TYRVAYA 1 time in each nostril, 2 times daily (about 12 hours apart).
- •
- A 1-month supply of TYRVAYA consists of 2 nasal spray bottles. Finish 1 bottle before opening the second. TYRVAYA comes in glass bottles with a white nasal pump and blue dust cover.
- •
- If you miss a dose of TYRVAYA, skip that dose and take your next dose at your regular scheduled time. Do not take an extra dose to make up for a missed dose.
What are the possible side effects of TYRVAYA?
The most common side effects of TYRVAYA include sneezing, cough, and throat and nose irritation.
These are not the only possible side effects of TYRVAYA. Call your doctor for medical advice about side effects.
You may report side effects to FDA at 1-800-FDA-1088.
How should I store TYRVAYA?
- •
- Store TYRVAYA at room temperature between 68°F to 77°F (20°C to 25°C).
- •
- Do not freeze.
- •
- Throw away (discard) TYRVAYA nasal spray bottle 30 days after first use.
Keep TYRVAYA and all medicines out of the reach of children.
General information about the safe and effective use of TYRVAYA.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use TYRVAYA for a condition for which it was not prescribed. Do not give TYRVAYA to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about TYRVAYA that is written for health professionals.
What are the ingredients in TYRVAYA?
Active ingredient: varenicline tartrate
Inactive ingredients: sodium phosphate dibasic heptahydrate, monobasic sodium phosphate anhydrous, sodium chloride, sodium hydroxide and/or hydrochloric acid (to adjust pH) and water for injection.
TYRVAYA® is a registered trademark of Oyster Point Pharma, Inc., a Viatris Company.
TYRVAYA® and/or the use of TYRVAYA® in a method may be covered by one or more patents or patent applications, available at www.oysterpointrx.com/patent-notices.
Manufactured for: Oyster Point Pharma, Inc., 202 Carnegie Center, Suite 106, Princeton, NJ 08540
This Patient Information has been approved by the U.S. Food and Drug Administration
Revised: 2/2024
OYP:PL:TYRVA:R1
-
Instructions for Use
TYRVAYA® (Teer-vye-ah)
(varenicline solution)
nasal spray, for intranasal useRead this Instructions for Use before you start using TYRVAYA and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
- •
-
Reprime: If you do not use TYRVAYA for more than 5 days, you will need to reprime the nasal spray bottle with 1 spray before you start using it. To reprime, hold the nasal spray bottle upright and away from your face and fully press and release the nasal spray applicator 1 time.
- •
- Avoid priming the nasal spray bottle more than needed: Priming the nasal spray bottle more than needed will reduce the amount of medicine in the nasal spray bottle.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
TYRVAYA® is a registered trademark of Oyster Point Pharma, Inc., a Viatris company.
TYRVAYA® and/or the use of TYRVAYA® in a method may be covered by one or more patents or patent applications, available at www.oysterpointrx.com/patent-notices.
Manufactured for: Oyster Point Pharma, Inc., 202 Carnegie Center, Suite 106, Princeton, NJ 08540
©2023 Oyster Point Pharma, Inc.
Revised: 2/2024
OYP:IFU:TYRVA:R1
-
PRINCIPAL DISPLAY PANEL - NASAL SPRAY 0.03 mg
NDC 73521-030-02
tyrvaya®
(varenicline solution)
nasal spray 0.03 mg2 nasal spray bottles
for a 30-day supplyEach bottle contains 60 sprays
(Net Content 4.2 mL per bottle)FOR INTRANASAL USE
Rx only.
Each 50 μL spray contains:
varenicline 0.03 mg
(equivalent to 0.05 mg
varenicline tartrate)Inactive ingredients:
sodium phosphate dibasic
heptahydrate, monobasic
sodium phosphate
anhydrous, sodium chloride,
sodium hydroxide and/or
hydrochloric acid (to adjust
pH) and water for injection.Manufactured for:
Oyster Point Pharma, Inc.
Princeton, NJ 085401-877-EYE-0123
Store Tyrvaya between
20°C to 25°C (68°F to 77°F).Do not freeze.
Do not shake the bottles.
Discard individual bottle of
Tyrvaya 30 days after first use.Keep Tyrvaya and all
medicines out of the reach
of children.02TRADCTN, Rev 2.0
IMPORTANT
Read enclosed Patient
Information and Instructions
for Use for priming, dosing,
and re-priming information.
PRIME BEFORE FIRST USE.
FOR INTRANASAL USE.
Tyrvaya, Oyster Point, and the respective logos
are registered trademarks of Oyster Point Pharma, Inc. -
INGREDIENTS AND APPEARANCE
TYRVAYA
varenicline sprayProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:73521-030 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VARENICLINE TARTRATE (UNII: 82269ASB48) (VARENICLINE - UNII:W6HS99O8ZO) VARENICLINE 0.03 mg in 0.05 mL Inactive Ingredients Ingredient Name Strength SODIUM PHOSPHATE, DIBASIC, HEPTAHYDRATE (UNII: 70WT22SF4B) SODIUM PHOSPHATE, MONOBASIC, ANHYDROUS (UNII: KH7I04HPUU) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73521-030-02 2 in 1 CARTON 10/20/2021 1 4.2 mL in 1 BOTTLE, PUMP; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC:73521-030-90 1 in 1 CARTON 10/20/2021 2 4.2 mL in 1 BOTTLE, PUMP; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA213978 10/20/2021 Labeler - Oyster Point Pharma, Inc. (080478750)