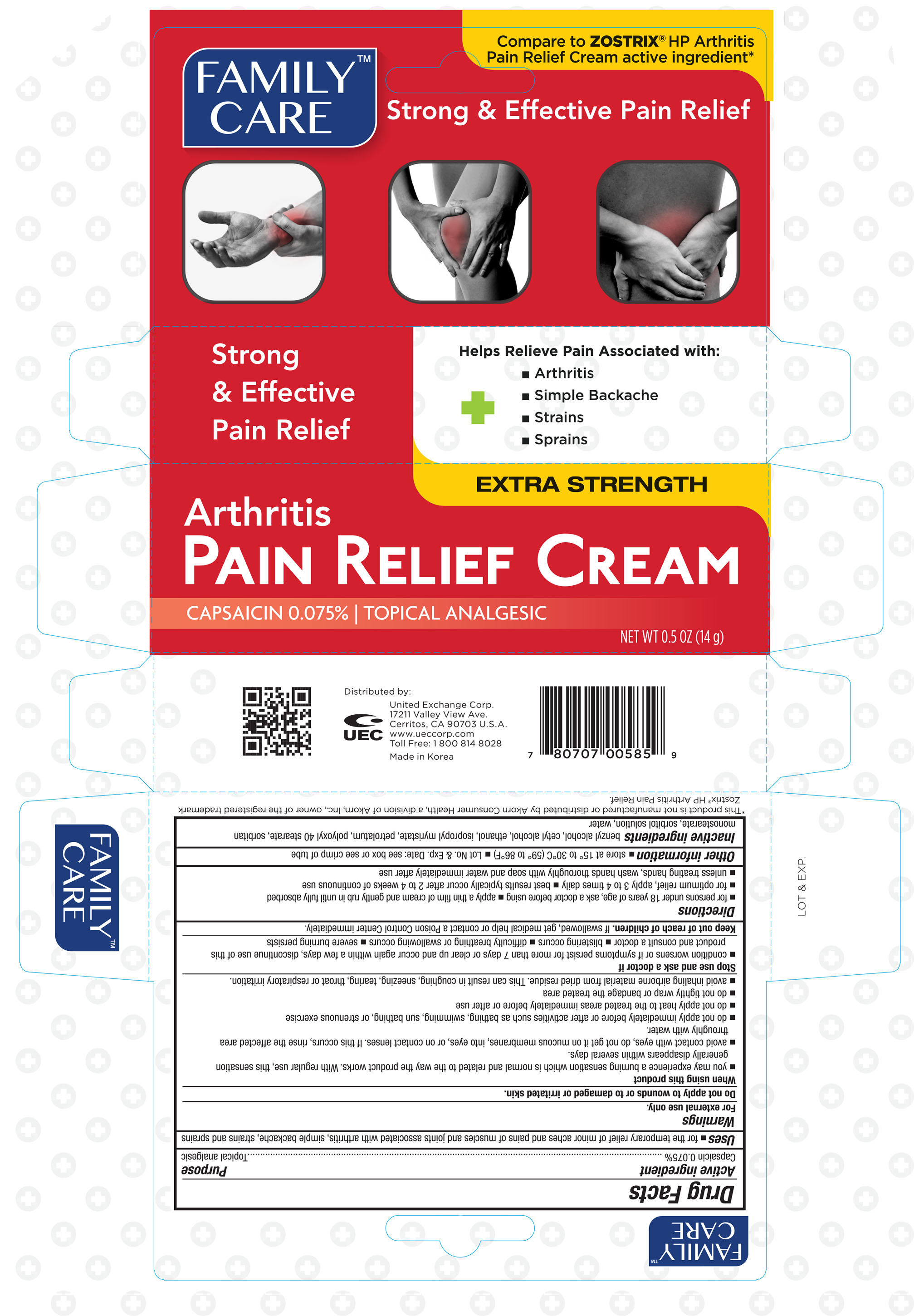
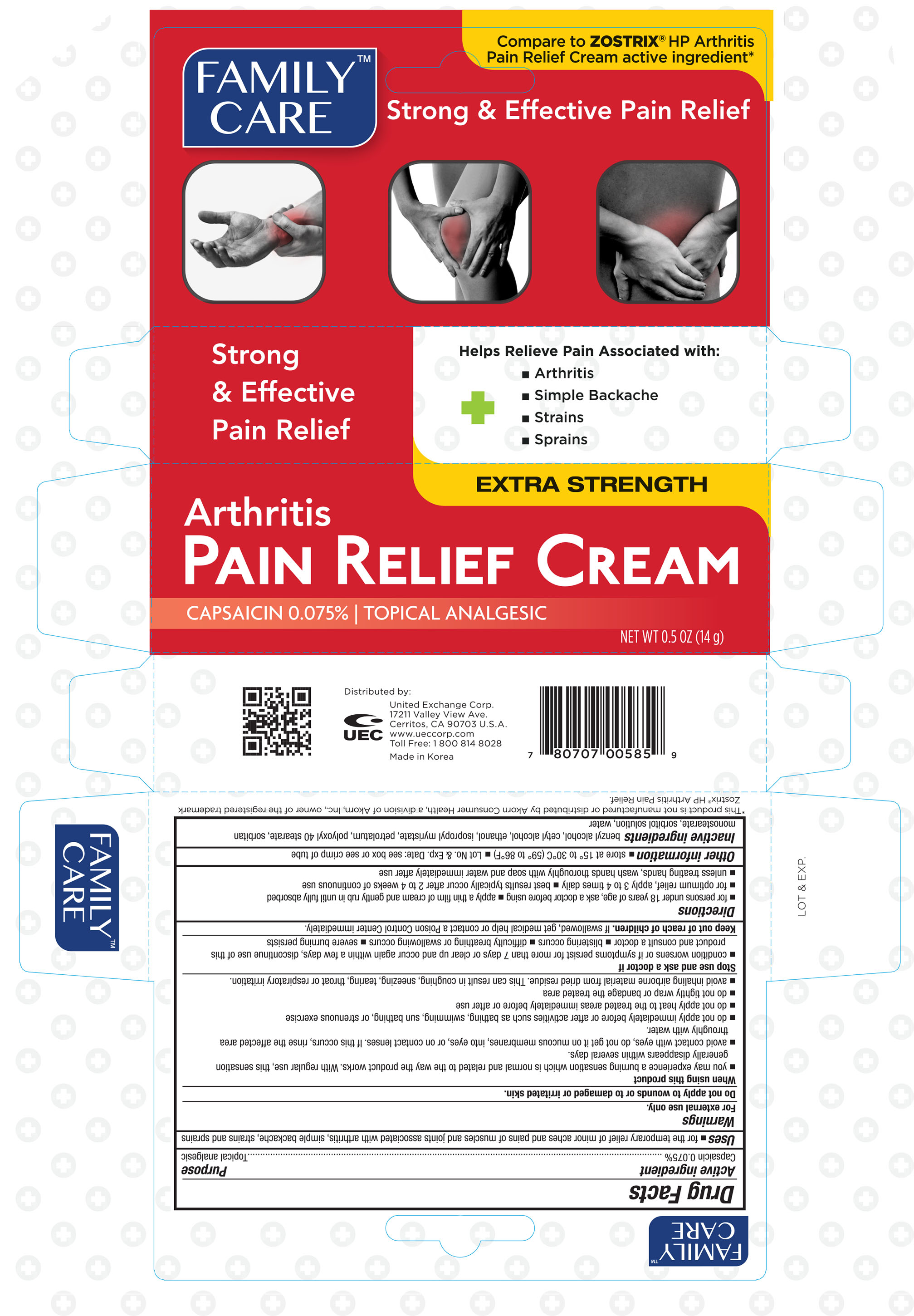
Label: FAMILY CARE ARTHRITIS PAIN RELIEF- capsaicin cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 65923-585-14 - Packager: United Excahnge Corp.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 26, 2016
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
-
WHEN USING
When using this product
- you may experience a burning sensation which is normal and related to the way the product works. With regular use, this sensation generally disapperas within several days.
- avoid contact with eyes, do not get on mucous membranes, into eyes, or on contact lesnes. If this occurs, rinse the affected area throughly with water.
- do not apply immediately before or after activities such as bathing, swimming, sun bathing, or strenuous excercise
- do not apply heat to the treated areas immediately before or after use
- do not tightly wrap or bandage the treated area
- avoid inhaling airborne material from dried residue. This can result in coughing, sneezing, tearing, throat or respiratory irritation.
- ASK DOCTOR
- KEEP OUT OF REACH OF CHILDREN
-
INDICATIONS & USAGE
Directions
- for persons under 18 years of age, ask a doctor before using
- apply a thin film of cream and gently rub in until fully absorbed
- for optimum relief, apply 3 to 4 times daily
- best results typically occur after 2 to 4 weeks of continuous use
- unless treating hands, wash hands throughly with soap and water immediately after use
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FAMILY CARE ARTHRITIS PAIN RELIEF
capsaicin creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65923-585 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAPSAICIN (UNII: S07O44R1ZM) (CAPSAICIN - UNII:S07O44R1ZM) CAPSAICIN 0.75 mg in 1 g Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) CETYL ALCOHOL (UNII: 936JST6JCN) ALCOHOL (UNII: 3K9958V90M) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) PETROLATUM (UNII: 4T6H12BN9U) POLYOXYL 40 STEARATE (UNII: 13A4J4NH9I) SORBITAN MONOSTEARATE (UNII: NVZ4I0H58X) SORBITOL (UNII: 506T60A25R) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65923-585-14 1 in 1 CARTON 07/26/2016 1 14 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 07/26/2016 Labeler - United Excahnge Corp. (840130579)