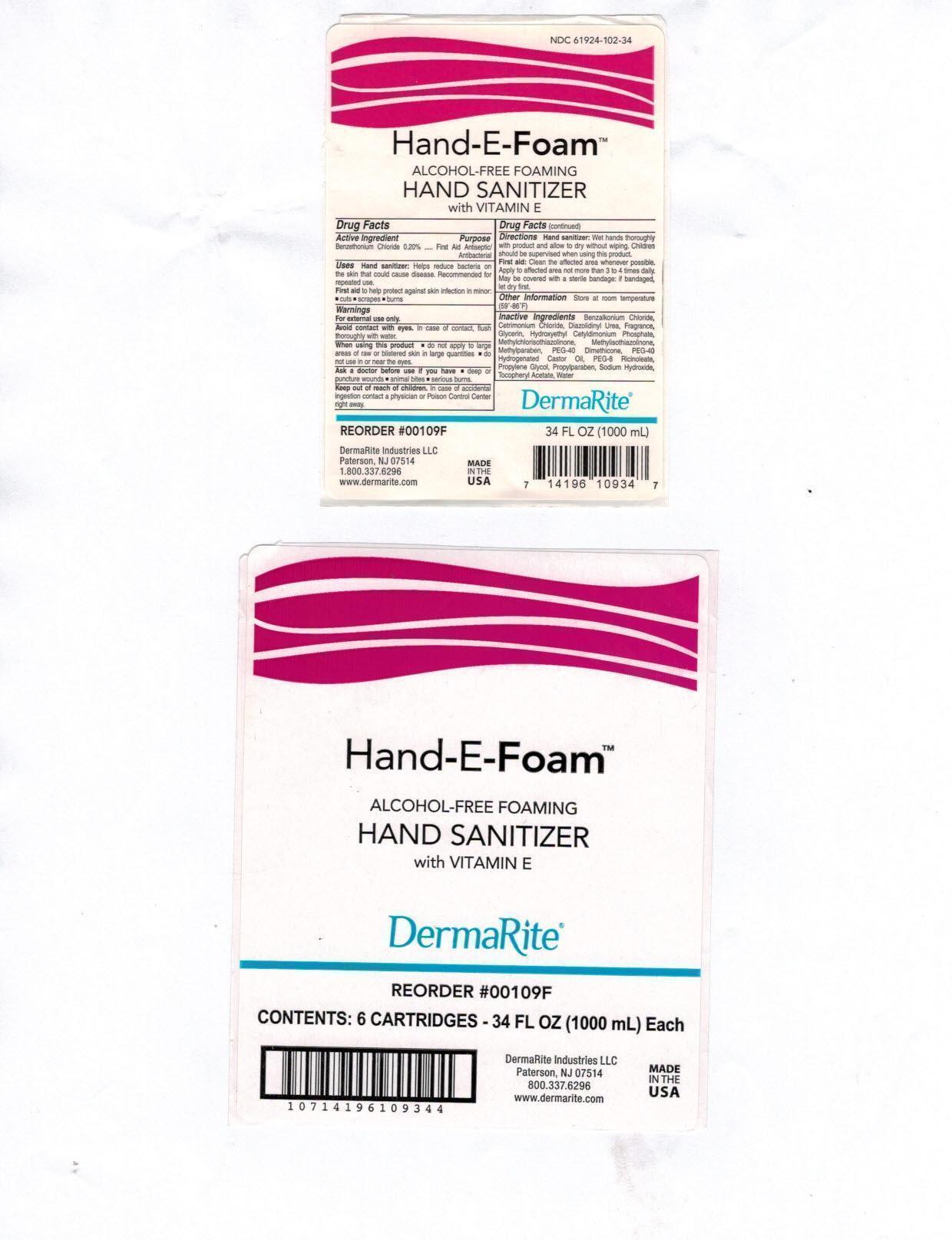
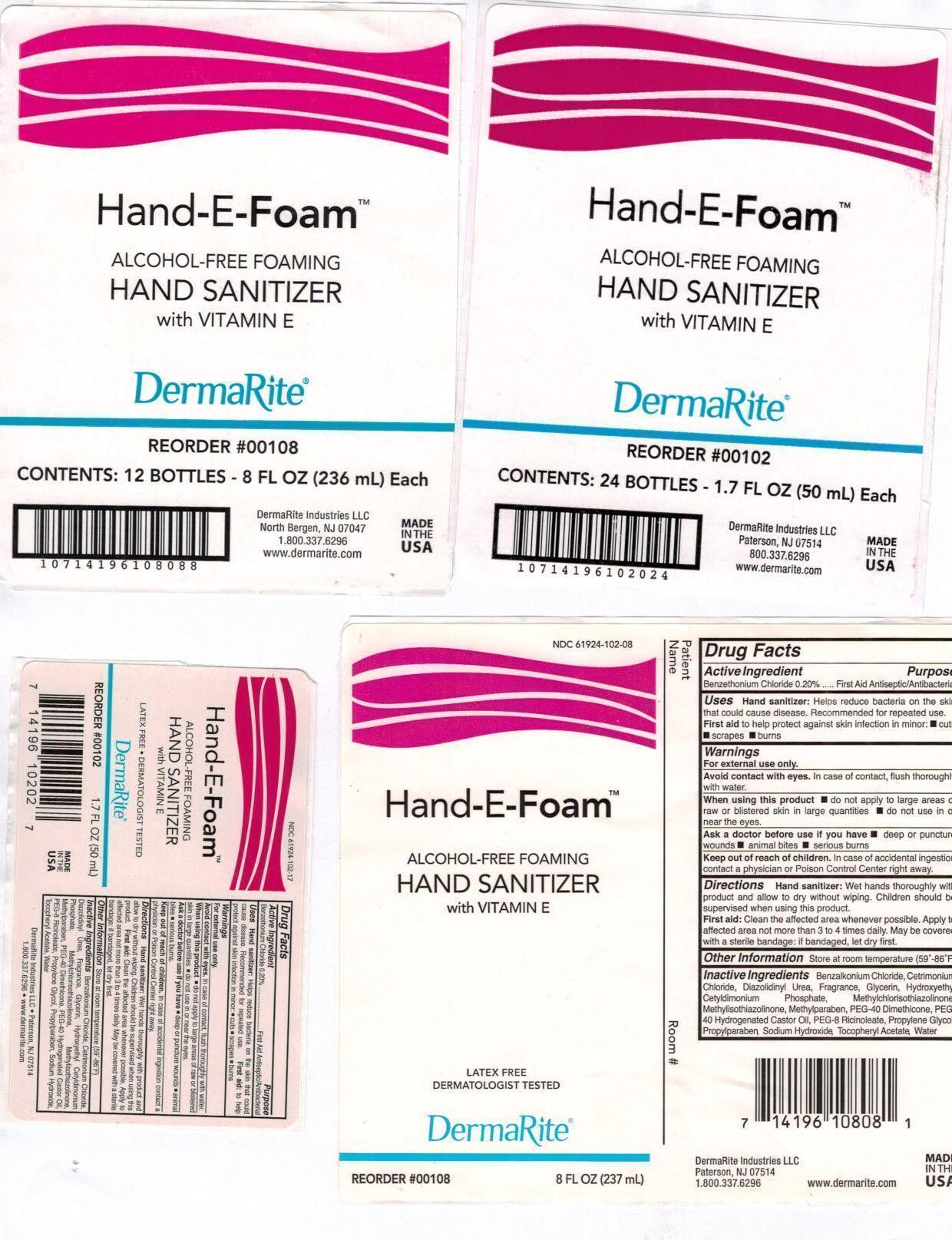
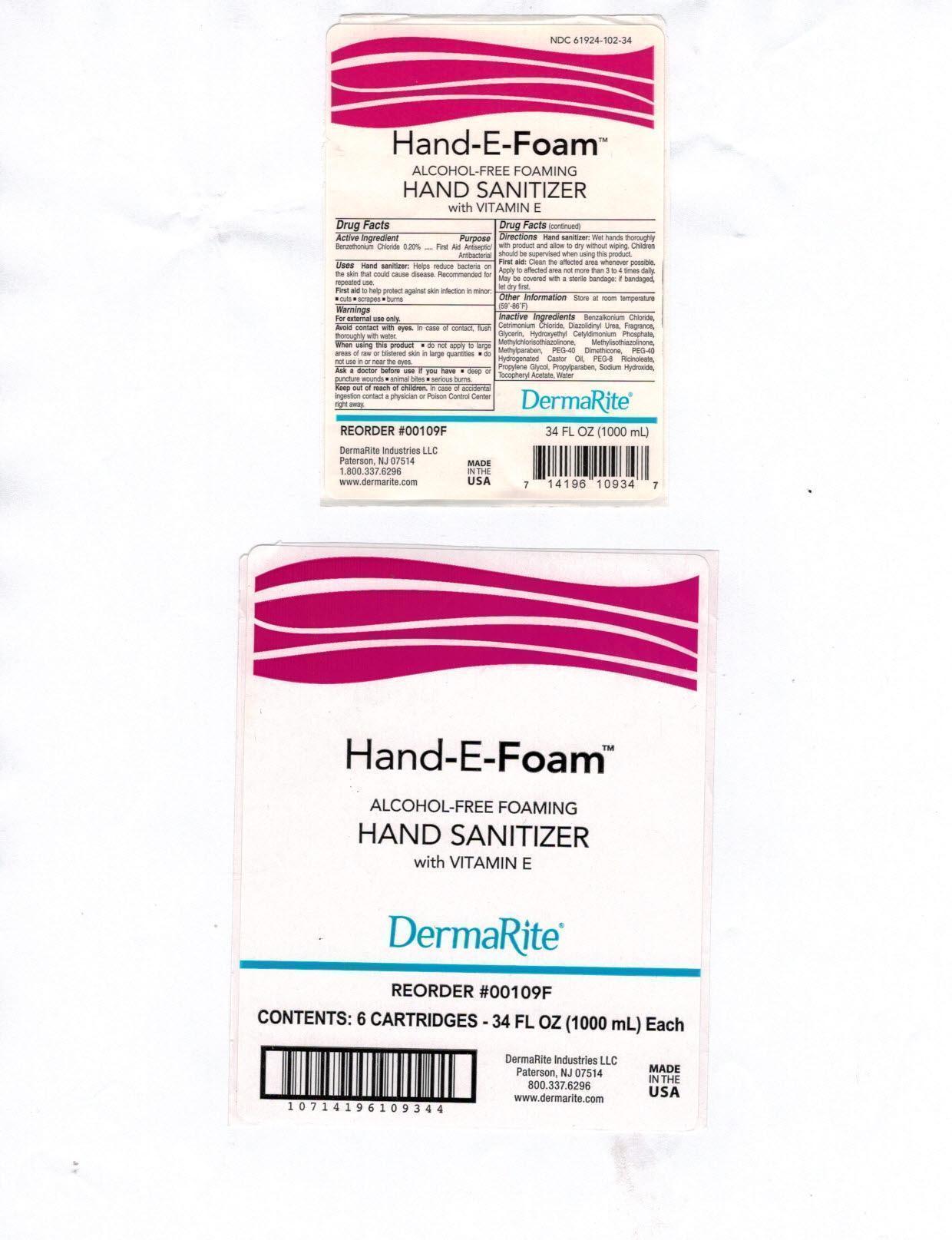
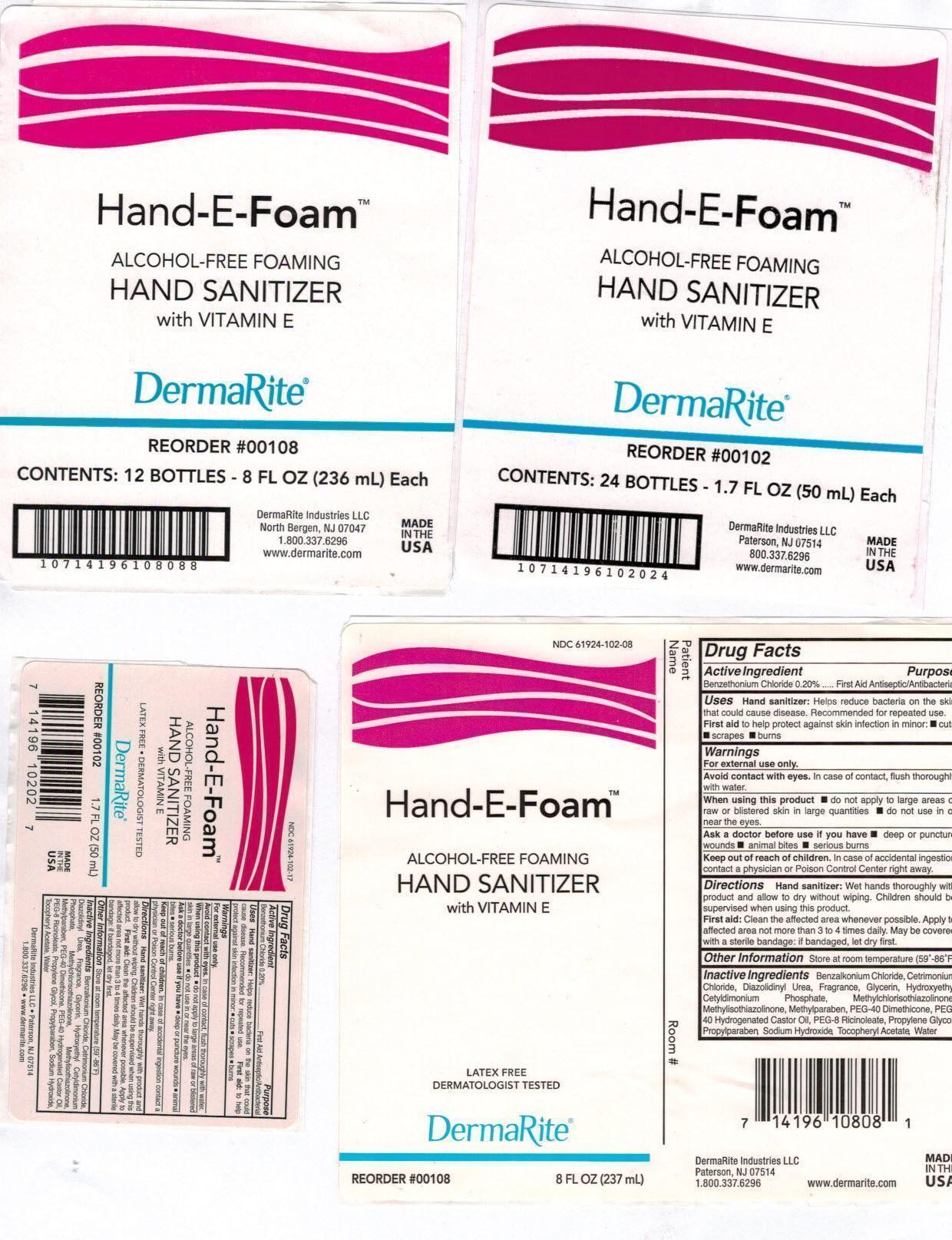
Label: HAND-E-FOAM- otc antimicrobial drug products aerosol, foam
-
Contains inactivated NDC Code(s)
NDC Code(s): 61924-102-08, 61924-102-17, 61924-102-34 - Packager: DermaRite Industries, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 14, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient:
- Purpose:
- Uses:
-
Warnings:
- For external use only.
- Avoid contact with eyes. In case of contact, flush thoroughly with water.
- When using this product do not apply to large area of raw or blistered skin in large quantities; do not use in or near the eyes.
-
Ask a doctor before use if you have , deep of puncture wounds, animal bites, serious burns
- Warnings
-
Directions:
- Hand sanitizer: Wet hands thoroughly with product and allow to dry without wiping. Children should be supervised when using this product.
- First Aid: Clean the affected area whenever possible. Apply to affected area not more than 3 to 4 times daily. May be covered with a sterile bandage:if bandaged, let dry first.
- Other Information:
-
Inactive Ingredients:
Benzalkonium Chloride, Cetrimonium Chloride, Diazolidinyl Urea, Fragrance, Glycerin, Hydroxethyl Cetyldimonium Phosphate, Methylchloroisothiazolinone, Methylisothiazolinone, Methylparaben, PEG-40 Dimethicone, PEG-40 Hydrogenated Castor Oil, PEG-8 Ricinoleate, Propylene Glycol, Propylparaben, Sodium Hydroxide, Tocopheryl Acetate, Water
- Hand-E-Foam Package Label Principal Display Panel
-
INGREDIENTS AND APPEARANCE
HAND-E-FOAM
otc antimicrobial drug products aerosol, foamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61924-102 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZETHONIUM CHLORIDE (UNII: PH41D05744) (BENZETHONIUM - UNII:1VU15B70BP) BENZETHONIUM CHLORIDE 0.002 g in 1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) CETRIMONIUM CHLORIDE (UNII: UC9PE95IBP) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) GLYCERIN (UNII: PDC6A3C0OX) HYDROXYETHYL CETYLDIMONIUM PHOSPHATE (UNII: 9G05UO431K) METHYLCHLOROISOTHIAZOLINONE (UNII: DEL7T5QRPN) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) METHYLPARABEN (UNII: A2I8C7HI9T) PEG-8 DIMETHICONE (UNII: GIA7T764OD) POLYOXYL 40 HYDROGENATED CASTOR OIL (UNII: 7YC686GQ8F) PEG-8 RICINOLEATE (UNII: DM36F4D2OU) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) SODIUM HYDROXIDE (UNII: 55X04QC32I) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61924-102-08 237 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 08/08/2002 2 NDC:61924-102-17 50 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 08/08/2002 3 NDC:61924-102-34 1000 mL in 1 CARTRIDGE; Type 0: Not a Combination Product 08/08/2002 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 08/08/2002 Labeler - DermaRite Industries, LLC (883925562) Registrant - DermaRite Industries, LLC (883925562) Establishment Name Address ID/FEI Business Operations DermaRite Industries, LLC 883925562 manufacture(61924-102)