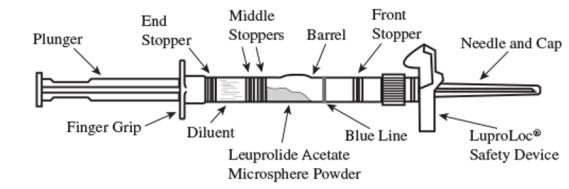
Label: LUPRON DEPOT- leuprolide acetate kit
- NDC Code(s): 0074-3641-03, 0074-3641-71
- Packager: AbbVie Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated October 27, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use LUPRON DEPOT 3.75 mg safely and effectively. See full prescribing information for LUPRON DEPOT 3.75 mg.
LUPRON DEPOT 3.75 mg (leuprolide acetate for depot suspension) for injection, for intramuscular use
Initial U.S. Approval: 1985
INDICATIONS AND USAGE
LUPRON DEPOT 3.75 mg is a gonadotropin-releasing hormone (GnRH) agonist indicated for:
Endometriosis
- Management of endometriosis, including pain relief and reduction of endometriotic lesions. (1.1)
- In combination with a norethindrone acetate for initial management of the painful symptoms of endometriosis and for management of recurrence of symptoms. (1.1)
Limitations of Use:
- The total duration of therapy with LUPRON DEPOT 3.75 mg plus add-back therapy should not exceed 12 months due to concerns about adverse impact on bone mineral density. (1.1, 2.1, 5.1)
Uterine Leiomyomata (Fibroids)
- Concomitant use with iron therapy for preoperative hematologic improvement of women with anemia caused by fibroids for whom three months of hormonal suppression is deemed necessary. (1.2)
Limitations of Use:
- LUPRON DEPOT 3.75 mg is not indicated for combination use with norethindrone acetate add-back therapy for the preoperative hematologic improvement of women with anemia caused by heavy menstrual bleeding due to fibroids. (1.2)
DOSAGE AND ADMINISTRATION
LUPRON DEPOT 3.75 mg for 1-month administration, given by a healthcare provider as a single intramuscular injection.
LUPRON DEPOT 3.75 mg has different release characteristics than LUPRON 11.25 mg and is dosed differently. (2.1)
- Do not substitute LUPRON DEPOT 3.75 mg for LUPRON DEPOT 11.25 mg.
- Do not administer LUPRON DEPOT 3.75 mg more frequently than once a month.
- Do not give a fractional dose of the LUPRON DEPOT 11.25 mg 3-month formulation, as it is not equivalent to a single dose of the LUPRON DEPOT 3.75 mg.
- Do not give a triple dose of the LUPRON DEPOT 3.75 mg, as it is not equivalent to a single dose of the LUPRON DEPOT 11.25 mg 3- month formulation.
Reconstitute LUPRON DEPOT 3.75 mg prior to use. (2.2)
Endometriosis:
- LUPRON DEPOT 3.75 mg administered as a single intramuscular (IM) injection once every month for up to six injections (6 months of therapy). LUPRON DEPOT may be administered alone or in combination with daily 5 mg tablet of norethindrone acetate (add-back). (2.1)
- If endometriosis symptoms recur after initial course of therapy, retreatment for no more than six months may be considered but only with the addition of norethindrone acetate add-back therapy. Do not re-treat with LUPRON DEPOT 3.75 mg alone. (2.1)
Fibroids:
- Recommended dose of LUPRON DEPOT 3.75 mg is one IM injection every month for up to three months. (2.1)
DOSAGE FORMS AND STRENGTHS
- Depot suspension for injection: 3.75 mg lyophilized powder for reconstitution in a dual-chamber syringe. (3)
CONTRAINDICATIONS
- Hypersensitivity to GnRH, GnRH agonist analogs, including leuprolide acetate, or any of the excipients in LUPRON DEPOT 3.75 mg. (4, 5.3)
- Undiagnosed abnormal uterine bleeding. (4)
- Pregnancy. (4, 8.1)
If LUPRON DEPOT 3.75 mg is administered with norethindrone acetate, the contraindications for norethindrone acetate also apply. (4)
WARNINGS AND PRECAUTIONS
- Loss of bone mineral density (BMD): Duration of treatment is limited by risk of bone mineral density. When using for management of endometriosis: combination use with norethindrone acetate is effective in reducing loss of BMD; do not retreat without combination norethindrone acetate. Assess BMD before retreatment. (1.1, 1.2, 5.1)
- Embryo-Fetal Toxicity: May cause fetal harm. Exclude pregnancy before initiating treatment if clinically indicated and discontinue use if pregnancy occurs. Use non-hormonal methods of contraception only. (5.2)
- Hypersensitivity reactions: Acute hypersensitivity, including anaphylaxis, have been reported with LUPRON DEPOT 3.75 mg. Delayed hypersensitivity have been very rarely reported in association with leuprolide-containing therapy. Discontinue future leuprolide therapy at first signs or symptoms of a delayed hypersensitivity reaction, and treat patients according to current treatment guidelines. (5.3)
- If LUPRON is administered with norethindrone acetate, the warnings and precautions for norethindrone acetate apply to the combination regimen. (5.7)
ADVERSE REACTIONS
Most common adverse reactions (>10%) in clinical trials were hot flashes/sweats, headache/migraine, vaginitis, depression/emotional lability, general pain, weight gain/loss, nausea/vomiting, decreased libido, and dizziness. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact AbbVie Inc. at 1-800-633-9110 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatchSee 17 for PATIENT COUNSELING INFORMATION.
Revised: 10/2023
- Management of endometriosis, including pain relief and reduction of endometriotic lesions. (1.1)
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Endometriosis
1.2 Uterine Leiomyomata (Fibroids)
2 DOSAGE AND ADMINISTRATION
2.1 Important Use Information
2.2 Reconstitution and Administration for Injection of LUPRON DEPOT
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Loss of Bone Mineral Density
5.2 Embryo-Fetal Toxicity
5.3 Hypersensitivity Reactions
5.4 Initial Flare of Symptoms
5.5 Convulsions
5.6 Clinical Depression
5.7 Risks Associated with Norethindrone Combination Treatment
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Endometriosis
14.2 Fibroids
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Endometriosis
Monotherapy
LUPRON DEPOT 3.75 mg is indicated for management of endometriosis, including pain relief and reduction of endometriotic lesions.
In Combination with Norethindrone Acetate
LUPRON DEPOT 3.75 mg in combination with norethindrone acetate is indicated for initial management of the painful symptoms of endometriosis and for management of recurrence of symptoms.
Use of norethindrone acetate in combination with LUPRON DEPOT 3.75 mg is referred to as add-back therapy, and is intended to reduce the loss of bone mineral density (BMD) and reduce vasomotor symptoms associated with use of LUPRON DEPOT 3.75 mg.
Limitations of Use:
The total duration of therapy with LUPRON DEPOT 3.75 mg plus add-back therapy should not exceed 12 months due to concerns about adverse impact on bone mineral density [see Dosage and Administration (2.1) and Warnings and Precautions (5.1)].
1.2 Uterine Leiomyomata (Fibroids)
LUPRON DEPOT 3.75 mg, used concomitantly with iron therapy, is indicated for the preoperative hematologic improvement of women with anemia caused by fibroids for whom three months of hormonal suppression is deemed necessary.
Consider a one-month trial period on iron alone, as some women will respond to iron alone [see Clinical Studies (14.2)]. LUPRON DEPOT 3.75 mg may be added if the response to iron alone is considered inadequate.
Limitations of Use:
LUPRON DEPOT 3.75 mg is not indicated for combination use with norethindrone acetate add-back therapy for the preoperative hematologic improvement of women with anemia caused by heavy menstrual bleeding due to fibroids [see Dosage and Administration (2.1)].
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Use Information
LUPRON DEPOT 3.75 mg must be administered by a healthcare professional.
LUPRON DEPOT 3.75 mg for 1-month administration has different release characteristics than LUPRON 11.25 mg for 3-month administration and is dosed differently.
- Do not substitute LUPRON DEPOT 3.75 mg for LUPRON DEPOT 11.25 mg.
- Do not administer LUPRON DEPOT 3.75 mg more frequently than once a month.
- Do not give a fractional dose of the LUPRON DEPOT 11.25 mg 3-month formulation as it is not equivalent to a single dose of the LUPRON DEPOT 3.75 mg.
- Do not give a triple dose of the LUPRON DEPOT 3.75 mg, as it is not equivalent to a single dose of the LUPRON DEPOT 11.25 mg 3-month formulation.
Endometriosis
The initial and retreatment dosage regimens for LUPRON DEPOT 3.75 mg for the management of women with endometriosis are outlined in Table 1.
Table 1. LUPRON DEPOT 3.75 mg, Management of Endometriosis Treatment Phase LUPRON DEPOT 3.75 mg Dosing Maximum Treatment Duration Initial Treatment1 3.75 mg IM every 1 month
for 1 to 6 doses6 months Retreatment2 3.75 mg IM every 1 month
for 1 to 6 doses6 months 12 MONTHS3
TOTAL TREATMENT DURATION1May use LUPRON DEPOT 3.75 mg with or without norethindrone acetate 5 mg tablet taken daily.
2Use LUPRON DEPOT 3.75 mg with norethindrone acetate for retreatment 5 mg tablet taken daily [see Warnings and Precautions (5.1)] and assess bone mineral density (BMD) prior to retreatment.
3Treatment should not exceed 12 months due to concerns about adverse impact on bone mineral density.
Fibroids
The recommended dosage of LUPRON DEPOT 3.75 mg is one IM injection every month for up to three months.
2.2 Reconstitution and Administration for Injection of LUPRON DEPOT
- Reconstitute and administer the lyophilized microsphere as a single IM injection as directed below. Visually inspect the drug product for particulate matter and discoloration prior to administration, whenever solution and container permit.
- Inject the LUPRON DEPOT 3.75 mg suspension immediately or discard if not used within two hours as the suspension does not contain a preservative.
1. Visually inspect the LUPRON DEPOT 3.75 mg powder. Do not use the syringe if clumping or caking is evident. A thin layer of powder on the wall of the syringe is considered normal prior to mixing with the diluent. The diluent should appear clear.
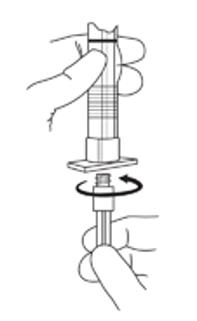
2. To prepare for injection, screw the white plunger into the end stopper until the stopper begins to turn (see Figure A and Figure B).
Figure A:
Figure B:
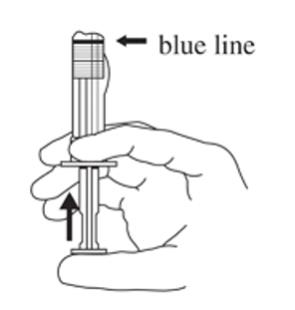
3. Hold the syringe UPRIGHT. Release the diluent by SLOWLY PUSHING the plunger for 6 to 8 seconds until the first middle stopper is at the blue line in the middle of the barrel (see Figure C).
Figure C:
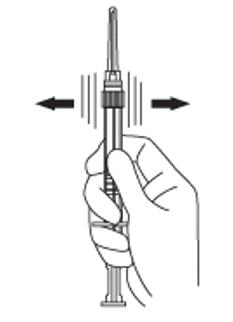
4. Keep the syringe upright. Mix the microsphere powder thoroughly by gently shaking the syringe until the powder forms a uniform suspension. The suspension will appear milky. If the powder adheres to the stopper or caking/clumping is present, tap the syringe with your finger to disperse. Do not use if any of the powder has not gone into suspension (see Figure D).
Figure D:
5. Keep the syringe upright. With the opposite hand pull the needle cap upward without twisting.
6. Keep the syringe upright. Advance the plunger to expel the air from the syringe. The syringe is now ready for injection.
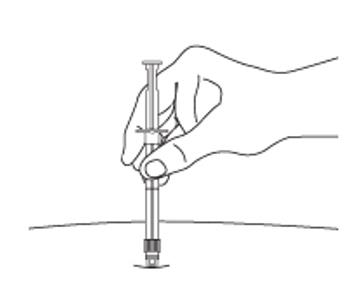
7. After cleaning the injection site with an alcohol swab, administer the IM injection by inserting the needle at a 90-degree angle into the gluteal area, anterior thigh, or deltoid. Injection sites should be alternated (see Figure E).
Figure E:
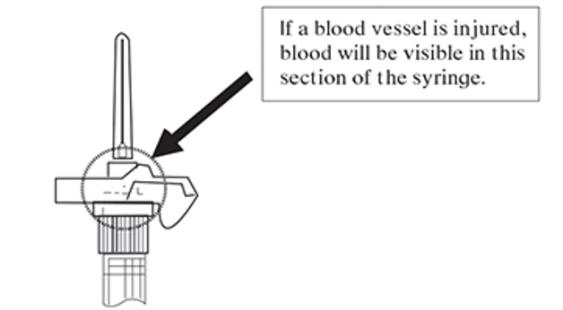
Note: If a blood vessel is accidentally penetrated, aspirated blood will be visible just below the luer lock (see Figure F) and can be seen through the transparent LuproLoc® safety device. If blood is present, remove the needle immediately. Do not inject the medication.
Figure F:
8. Inject the entire contents of the syringe intramuscularly.
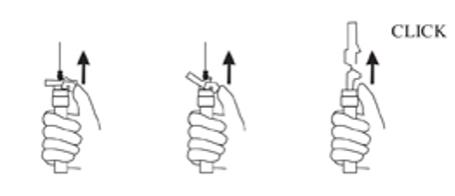
9. Withdraw the needle. Once the syringe has been withdrawn, immediately activate the LuproLoc® safety device by pushing the arrow on the lock upward towards the needle tip with the thumb or finger, as illustrated, until the needle cover of the safety device over the needle is fully extended and a click is heard or felt (see Figure G).
Figure G:
10. Dispose of the syringe according to local regulations/procedures.
- Do not substitute LUPRON DEPOT 3.75 mg for LUPRON DEPOT 11.25 mg.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
LUPRON DEPOT 3.75 mg is contraindicated in women with the following:
- Hypersensitivity to gonadotropin-releasing hormone (GnRH), GnRH agonist analogs, including leuprolide acetate, or any of the excipients in LUPRON DEPOT 3.75 mg [see Warnings and Precautions (5.3) and Adverse Reactions (6.2)]
- Undiagnosed abnormal uterine bleeding
- Pregnancy [see Warnings and Precautions (5.2) and Use in Specific Populations (8.1)]
When norethindrone acetate is administered with LUPRON DEPOT 3.75 mg, the contraindications to the use of norethindrone acetate also apply to this combination regimen. Refer to the norethindrone acetate prescribing information for a list of contraindications for norethindrone acetate.
- Hypersensitivity to gonadotropin-releasing hormone (GnRH), GnRH agonist analogs, including leuprolide acetate, or any of the excipients in LUPRON DEPOT 3.75 mg [see Warnings and Precautions (5.3) and Adverse Reactions (6.2)]
-
5 WARNINGS AND PRECAUTIONS
5.1 Loss of Bone Mineral Density
LUPRON DEPOT 3.75 mg induces a hypoestrogenic state that results in loss of bone mineral density (BMD), some of which may not be reversible after stopping treatment. In women with major risk factors for decreased BMD such as chronic alcohol use (> 3 units per day), tobacco use, strong family history of osteoporosis, or chronic use of drugs that can decrease BMD, such as anticonvulsants or corticosteroids, use of LUPRON DEPOT 3.75 mg may pose an additional risk. Carefully weigh the risks and benefits of LUPRON DEPOT 3.75 mg use in these populations.
The duration of LUPRON DEPOT 3.75 mg treatment is limited by the risk of loss of bone mineral density [see Dosage and Administration (2.1)].
When using LUPRON DEPOT 3.75 mg for the management of endometriosis, combination use of norethindrone acetate (add-back therapy) is effective in reducing the loss of BMD that occurs with leuprolide acetate [see Clinical Studies (14.2)]. Do not retreat with LUPRON DEPOT 3.75 mg without combination norethindrone acetate. Assess BMD before retreatment.
5.2 Embryo-Fetal Toxicity
Based on animal reproduction studies and the drug’s mechanism of action, LUPRON DEPOT 3.75 mg may cause fetal harm if administered to a pregnant woman and is contraindicated in pregnant women. Exclude pregnancy prior to initiating treatment with LUPRON DEPOT 3.75 mg if clinically indicated. Discontinue LUPRON DEPOT 3.75 mg if the woman becomes pregnant during treatment and inform the woman of potential risk to the fetus [see Contraindications (4) and Use in Specific Populations (8.1)]. Advise women to notify their healthcare provider if they believe they may be pregnant.
When used at the recommended dose and dosing interval, LUPRON DEPOT 11.25 mg usually inhibits ovulation and stops menstruation. Contraception, however, is not ensured by taking LUPRON DEPOT 11.25 mg. If contraception is indicated, advise women to use non-hormonal methods of contraception while on treatment with LUPRON DEPOT 3.75 mg.
5.3 Hypersensitivity Reactions
Acute Hypersensitivity
Acute hypersensitivity reactions, including anaphylaxis, have been reported with LUPRON DEPOT use. LUPRON DEPOT 3.75 mg is contraindicated in women with a history of hypersensitivity to gonadotropin-releasing hormone (GnRH) or GnRH agonist analogs [see Contraindications (4) and Adverse Reactions (6.2)].
In clinical trials of LUPRON DEPOT 3.75 mg, adverse events of asthma were reported in women with pre-existing histories of asthma, sinusitis, and environmental or drug allergies. Symptoms consistent with an anaphylactoid or asthmatic process have been reported postmarketing.
Delayed Hypersensitivity
Delayed hypersensitivity reactions including the severe cutaneous adverse reactions (SCAR) of Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) have been very rarely reported post-marketing in association with leuprolide-containing therapy [see Adverse Reactions (6.2)]. Discontinue future leuprolide therapy at first signs or symptoms of a delayed hypersensitivity reaction, and treat patients according to current treatment guidelines.
5.4 Initial Flare of Symptoms
Following the first dose of LUPRON DEPOT 3.75 mg, sex steroids temporarily rise above baseline because of the physiologic effect of the drug. Therefore, an increase in symptoms may be observed during the initial days of therapy, but these should dissipate with continued therapy.
5.5 Convulsions
There have been postmarketing reports of convulsions in women on GnRH agonists, including leuprolide acetate. These included women with and without concurrent medications and comorbid conditions.
5.6 Clinical Depression
Depression may occur or worsen during treatment with GnRH agonists including LUPRON DEPOT 3.75 mg [see Adverse Reactions (6.1)]. Carefully observe women for depression, especially those with a history of depression and consider whether the risks of continuing LUPRON DEPOT 3.75 mg outweigh the benefits. Women with new or worsening depression should be referred to a mental health professional, as appropriate.
5.7 Risks Associated with Norethindrone Combination Treatment
If LUPRON DEPOT 3.75 mg is administered with norethindrone acetate, the warnings and precautions for norethindrone acetate apply to this regimen. Refer to the norethindrone acetate prescribing information for a full list of the warnings and precautions for norethindrone acetate.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Loss of Bone Mineral Density [see Warnings and Precautions (5.1)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.3)]
- Initial Flare of Symptoms with Management of Endometriosis [see Warnings and Precautions (5.4)]
- Convulsions [see Warnings and Precautions (5.5)]
- Clinical Depression [see Warnings and Precautions (5.6)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
LUPRON DEPOT 3.75 mg (Monotherapy)
The safety of LUPRON DEPOT 3.75 mg for the endometriosis and fibroids indications was established based on adequate and well-controlled adult studies. The safety of LUPRON DEPOT 3.75 mg was evaluated in six clinical studies in which a total of 332 women were treated for up to six months. Women were treated with monthly IM injections of LUPRON DEPOT 3.75 mg. The population age range was 18 to 53 years old.
Adverse Reactions (>1%) Leading to Study Discontinuation
In the six studies 1.8% of women treated with LUPRON DEPOT 3.75 mg discontinued prematurely due to hot flashes.
Common Adverse Reactions
The safety of LUPRON DEPOT 3.75 mg was evaluated in controlled clinical trials in 166 women with endometriosis and 166 women with uterine fibroids. Adverse reactions reported in ≥ 5% of women in either of these populations are noted in Tables 2 and 3, below.
Table 2. Adverse Reactions Reported in ≥ 5% of Women with Endometriosis Taking LUPRON DEPOT 3.75 mg - 2 Studies LUPRON DEPOT
3.75 mg
N=166Danazol
N=136Placebo
N=31% % % Hot flashes/sweats* 84 57 29 Headache* 32 22 6 Vaginitis* 28 17 0 Depression/emotional lability* 22 20 3 General pain 19 16 3 Weight gain/loss 13 26 0 Nausea/vomiting 13 13 3 Decreased libido* 11 4 0 Dizziness 11 3 0 Acne 10 20 0 Skin reactions 10 15 3 Joint disorder* 8 8 0 Edema 7 13 3 Paresthesias 7 8 0 GI disturbances* 7 6 3 Neuromuscular disorders* 7 13 0 Breast changes/tenderness/pain* 6 9 0 Nervousness* 5 8 0 In these same studies, symptoms reported in < 5% of women included: -
Body as a Whole - Injection site reactions
-
Cardiovascular System - Palpitations, syncope, tachycardia
-
Digestive System - Appetite changes, dry mouth, thirst
-
Endocrine System - Androgen-like effects, lactation
-
Blood and Lymphatic System - Ecchymosis
-
Nervous/Psychiatric System - Anxiety*, insomnia/sleep disorders*, delusions, memory disorder, personality disorder
-
Dermal System - Alopecia, hair disorder
-
Ocular system - Ophthalmologic disorders*
-
Urogenital System - Dysuria*.
* = Possible effect of decreased estrogen.
Table 3. Adverse Reactions Reported in ≥ 5% of Women with Uterine Fibroids (4 Studies) Taking LUPRON DEPOT 3.75 mg LUPRON DEPOT 3.75 mg
N=166Placebo
N=163% % Hot flashes/sweats* 73 18 Headache* 26 18 Vaginitis* 11 2 Depression/emotional lability* 11 4 Asthenia 8 5 General pain 8 6 Joint disorder* 8 3 Edema 5 1 Nausea/vomiting 5 4 Nervousness* 5 1 In these same studies, symptoms reported in < 5% of women included: -
Body as a Whole - Body odor, flu syndrome, injection site reactions
-
Cardiovascular System - Tachycardia
-
Digestive System - Appetite changes, dry mouth, taste perversion
-
Endocrine System - Androgen-like effects, menstrual disorders
-
Nervous/Psychiatric System - Anxiety*, insomnia/sleep disorders*
-
Respiratory System - Rhinitis
-
Dermal System - Nail disorder
-
Ocular system - Conjunctivitis
* = Possible effect of decreased estrogen.
In one controlled clinical trial utilizing the monthly formulation of LUPRON DEPOT 3.75 mg and LUPRON DEPOT 7.5 mg in women diagnosed with uterine fibroids received one injection every 4 weeks for a duration of 12 weeks. Adverse reactions of galactorrhea, pyelonephritis, and urinary incontinence were reported in the 7.5 mg dose group but not in the 3.75 mg dose group. Generally, a higher incidence of hypoestrogenic effects was observed at the higher dose.
LUPRON DEPOT 3.75 mg in combination with Norethindrone Acetate 5 mg
The safety of co-administering LUPRON DEPOT 3.75 mg and norethindrone acetate was evaluated in two clinical studies in which a total of 242 women with endometriosis were treated for up to one year. Women were treated with monthly IM injections of LUPRON DEPOT 3.75 mg (13 injections) alone or monthly IM injections of LUPRON DEPOT 3.75 mg (13 injections) plus norethindrone acetate 5 mg daily. The population age range was 17 to 43 years old. The majority of women were Caucasian (87%).
In one study, 106 women were randomized to one year of treatment with LUPRON DEPOT 3.75 mg alone or with LUPRON DEPOT 3.75 mg and norethindrone acetate. The other study was an open-label, single arm clinical study in 136 women on one year of treatment with LUPRON DEPOT 3.75 mg plus norethindrone acetate, with follow-up for up to 12 months after completing treatment.
Adverse Reactions (>1%) Leading to Study Discontinuation
In the controlled study, 18% of women treated monthly with LUPRON DEPOT 3.75 mg and 18% of women treated monthly with LUPRON DEPOT 3.75 mg plus norethindrone acetate discontinued therapy due to adverse reactions, most commonly hot flashes (6%) and insomnia (4%) in the LUPRON DEPOT 3.75 mg alone group and hot flashes and emotional lability (4% each) in the LUPRON DEPOT 3.75 mg plus norethindrone group.
In the open-label study, 13% of women treated monthly with LUPRON DEPOT 3.75 mg plus norethindrone acetate discontinued therapy due to adverse reactions, most commonly depression (4%) and acne (2%).
Common Adverse Reactions
Table 4 lists the adverse reactions observed in at least 5% of women in any treatment group, during the first 6 months of treatment in the two add-back clinical studies, in which women were treated with monthly LUPRON DEPOT 3.75 mg with or without norethindrone acetate 5 mg daily co-treatment. The most frequently-occurring adverse reactions observed in these studies were hot flashes and headaches.
Table 4. Adverse Reactions Occurring in the First Six Months of Treatment in ≥ 5% of Women with Endometriosis Controlled
StudyOpen Label
StudyLD-Only* LD/N† LD/N† N=51 N=55 N=136 Adverse Reactions % % % Any Adverse Reaction 98 96 93 Hot flashes/Sweats 98 87 57 Headache/Migraine 65 51 46 Depression/Emotional Lability 31 27 34 Insomnia/Sleep Disorder 31 13 15 Nausea/Vomiting 25 29 13 Pain 24 29 21 Vaginitis 20 15 8 Asthenia 18 18 11 Dizziness/Vertigo 16 11 7 Altered Bowel Function (constipation, diarrhea) 14 15 10 Weight Gain 12 13 4 Decreased Libido 10 4 7 Nervousness/Anxiety 8 4 11 Breast Changes/Pain/Tenderness 6 13 8 Memory Disorder 6 2 4 Skin/Mucous Membrane Reaction 4 9 11 GI Disturbance (dyspepsia, flatulence) 4 7 4 Androgen-Like Effects (acne, alopecia) 4 5 18 Changes in Appetite 4 0 6 Injection Site Reaction 2 9 3 Neuromuscular Disorder (leg cramps, paresthesia) 2 9 3 Menstrual Disorders 2 0 5 Edema 0 9 7 * LD-Only = LUPRON DEPOT 3.75 mg
† LD/N = LUPRON DEPOT 3.75 mg plus norethindrone acetate 5 mgIn the controlled clinical trial, 50 of 51 (98%) women in the LUPRON DEPOT 3.75 mg arm and 48 of 55 (87%) women in the LUPRON DEPOT 3.75 mg plus norethindrone acetate arm reported experiencing hot flashes on one or more occasions during treatment.
Table 5 presents hot flash data in the last month of treatment.
Table 5. Hot Flashes in the Month Prior to the Assessment Visit (Controlled Study) Assessment
VisitTreatment
GroupNumber of Women
Reporting Hot FlashesNumber of Days
with Hot FlashesMaximum Number
Hot Flashes in 24 HoursN (%) N2 Mean N2 Mean Week 24 LD-Only* 32/37 86 37 19 36 5.8 LD/N† 22/38 581 38 71 38 1.91 * LD-Only = LUPRON DEPOT 3.75 mg.
† LD/N = LUPRON DEPOT 3.75 mg plus norethindrone acetate 5 mg.
1Statistically significantly less than the LD-Only group (p<0.01).
2Number of women assessed.Serious Adverse Reactions
Urinary tract infection (1.9%), renal calculus (0.7%), depression (0.7%)
Changes in Laboratory Values during Treatment
Liver Enzymes
Three percent of women with uterine fibroids treated with LUPRON DEPOT 3.75 mg, manifested post-treatment transaminase values that were at least twice the baseline value and above the upper limit of the normal range.
In the two clinical trials of women with endometriosis, 2% (4 of 191) women receiving leuprolide acetate plus norethindrone acetate for up to 12 months developed an elevated (at least twice the upper limit of normal) SGPT and 1% (2 of 136) developed an elevated GGT. Among these six women with increased liver tests, the increases in five were observed beyond 6 months of treatment. None were associated with an elevated bilirubin concentration.
Lipids
Triglycerides were increased above the upper limit of normal in 12% of the women with endometriosis who received LUPRON DEPOT 3.75 mg.
Of those women with endometriosis and women with uterine fibroid whose pretreatment cholesterol values were in the normal range, mean change following therapy was +16 mg/dL to +17 mg/dL in women with endometriosis and +11 mg/dL to +29 mg/dL in women with uterine fibroids. In the women with endometriosis, increases from the pretreatment values were statistically significant (p<0.03). There was essentially no increase in the LDL/HDL ratio in women from either population receiving LUPRON DEPOT 3.75 mg.
Percent changes from baseline for serum lipids and percentages of women with serum lipid values outside of the normal range in the two studies of LUPRON DEPOT 3.75 mg and norethindrone acetate are summarized in Table 6 and Table 7 below. The major impact of adding norethindrone acetate to treatment with LUPRON DEPOT 3.75 mg was a decrease in serum HDL cholesterol and an increase in the LDL/HDL ratio.
Table 6. Serum Lipids: Mean Percent Changes from Baseline Values at Treatment Week 24 LUPRON DEPOT
3.75 mgLUPRON DEPOT 3.75 mg
plus norethindrone acetate 5 mg dailyControlled Study
(n=39)Controlled Study
(n=41)Open Label Study
(n=117)Baseline
Value*Week 24
% ChangeBaseline
Value*Week 24
% ChangeBaseline
Value*Week 24
% ChangeTotal Cholesterol 170.5 9.2% 179.3 0.2% 181.2 2.8% HDL Cholesterol 52.4 7.4% 51.8 -18.8% 51.0 -14.6% LDL Cholesterol 96.6 10.9% 101.5 14.1% 109.1 13.1% LDL/HDL Ratio 2.0† 5.0% 2.1† 43.4% 2.3† 39.4% Triglycerides 107.8 17.5% 130.2 9.5% 105.4 13.8% * mg/dL
† ratioChanges from baseline tended to be greater at Week 52. After treatment, mean serum lipid levels from women with follow-up data returned to pretreatment values.
Table 7. Percentage of Women with Serum Lipids Values Outside of the Normal Range LUPRON DEPOT
3.75 mgLUPRON DEPOT 3.75 mg
plus norethindrone acetate 5 mg dailyControlled Study
(n=39)Controlled Study
(n=41)Open Label Study
(n=117)Week 0 Week 24* Week 0 Week 24* Week 0 Week 24* Total Cholesterol (>240 mg/dL) 15% 23% 15% 20% 6% 7% HDL Cholesterol (<40 mg/dL) 15% 10% 15% 44% 15% 41% LDL Cholesterol (>160 mg/dL) 0% 8% 5% 7% 9% 11% LDL/HDL Ratio (>4.0) 0% 3% 2% 15% 7% 21% Triglycerides (>200 mg/dL) 13% 13% 12% 10% 5% 9% * Includes all women regardless of baseline value. 6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of LUPRON DEPOT monotherapy or LUPRON DEPOT with norethindrone acetate add-back therapy. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
During postmarketing surveillance which includes other dosage forms and other populations, the following adverse reactions were reported:
-
Body as a whole: Hypersensitivity reactions including anaphylaxis, localized reactions including induration and abscess at the site of injection
-
Nervous/Psychiatric System: Mood swings, including depression; suicidal ideation and attempt; convulsion, peripheral neuropathy, paralysis
-
Hepato-biliary system: Serious liver injury
-
Skin reactions: erythema multiforme, dermatitis bullous, dermatitis exfoliative, Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN)
-
Injury, poisoning and procedural complications: Spinal fracture
-
Investigations: Decreased white blood count
-
Musculoskeletal and connective tissue system: Tenosynovitis-like symptoms
-
Vascular system: Hypotension, hypertension, deep vein thrombosis, pulmonary embolism, myocardial infarction, stroke, transient ischemic attack
-
Respiratory system: Symptoms consistent with an asthmatic process
- Multi-system disorders: Symptoms consistent with fibromyalgia (e.g., joint and muscle pain, headaches, sleep disorders, gastrointestinal distress, and shortness of breath), individually and collectively.
Pituitary apoplexy
During postmarketing surveillance, cases of pituitary apoplexy (a clinical syndrome secondary to infarction of the pituitary gland) have been reported after the administration of leuprolide acetate and other GnRH agonists. In a majority of these cases, a pituitary adenoma was diagnosed, with a majority of pituitary apoplexy cases occurring within 2 weeks of the first dose, and some within the first hour. In these cases, pituitary apoplexy has presented as sudden headache, vomiting, visual changes, ophthalmoplegia, altered mental status, and sometimes cardiovascular collapse. Immediate medical attention has been required.
- Loss of Bone Mineral Density [see Warnings and Precautions (5.1)]
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
LUPRON DEPOT 3.75 mg is contraindicated in pregnancy [see Contraindications (4)].
LUPRON DEPOT 3.75 mg may cause fetal harm based on findings from animal studies and the drug’s mechanism of action [see Clinical Pharmacology (12.1)]. There are limited human data on the use of LUPRON DEPOT in pregnant women. Based on animal reproduction studies, LUPRON DEPOT 3.75 mg may be associated with an increased risk of pregnancy complications, including early pregnancy loss and fetal harm. In animal reproduction studies, subcutaneous administration of leuprolide acetate to rabbits during the period of organogenesis caused embryo-fetal toxicity, decreased fetal weights and a dose-dependent increase in major fetal abnormalities in animals at doses less than the recommended human dose based on body surface area using an estimated daily dose. A similar rat study also showed increased fetal mortality and decreased fetal weights but no major fetal abnormalities at doses less than the recommended human dose based on body surface area using an estimated daily dose [see Data].
Data
Animal Data
When administered on day 6 of pregnancy at test dosages of 0.00024 mg/kg, 0.0024 mg/kg, and 0.024 mg/kg (1/300 to 1/3 of the human dose) to rabbits, leuprolide acetate produced a dose-related increase in major fetal abnormalities. Similar studies in rats failed to demonstrate an increase in fetal malformations. There was increased fetal mortality and decreased fetal weights with the two higher doses of LUPRON DEPOT in rabbits and with the highest dose (0.024 mg/kg) in rats.
8.2 Lactation
Risk Summary
There are no data on the presence of leuprolide acetate in either animal or human milk, the effects on the breastfed infants, or the effects on milk production.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for LUPRON DEPOT 3.75 mg and any potential adverse effects on the breastfed infant from LUPRON DEPOT 3.75 mg or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Exclude pregnancy in women of reproductive potential prior to initiating LUPRON DEPOT 3.75 mg if clinically indicated [see Warnings and Precautions (5.2)].
Contraception
Females
LUPRON DEPOT 3.75 mg may cause embryo-fetal harm when administered during pregnancy. LUPRON DEPOT 3.75 mg is not a contraceptive. If contraception is indicated, advise females of reproductive potential to use a non-hormonal method of contraception during treatment with LUPRON DEPOT 3.75 mg [see Warnings and Precautions (5.2)].
Infertility
Based on its pharmacodynamic effects of decreasing secretion of gonadal steroids, fertility is expected to be decreased while on treatment with LUPRON DEPOT 3.75 mg. Clinical and pharmacologic studies in adults (>18 years) with leuprolide acetate and similar analogs have shown reversibility of fertility suppression when the drug is discontinued after continuous administration for periods of up to 24 weeks [see Clinical Pharmacology (12.1)].
There is no evidence that pregnancy rates are affected following discontinuation of LUPRON DEPOT 3.75 mg.
Animal studies (prepubertal and adult rats and monkeys) with leuprolide acetate and other GnRH analogs have shown functional recovery of fertility suppression.
8.4 Pediatric Use
Safety and effectiveness of LUPRON DEPOT 3.75 mg for management of endometriosis and the preoperative hematologic improvement of women with anemia caused by fibroids have been established in females of reproductive age. Efficacy is expected to be the same for postpubertal adolescents under the age of 18 as for users 18 years and older. The safety and effectiveness of LUPRON DEPOT 3.75 mg for these indications have not been established in premenarcheal pediatric patients.
-
11 DESCRIPTION
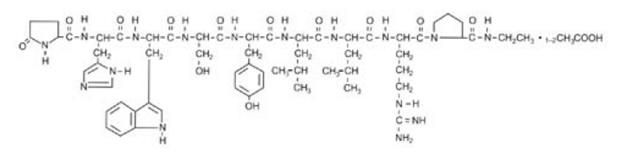
Leuprolide acetate is a synthetic nonapeptide analog of gonadotropin-releasing hormone [GnRH or luteinizing hormone releasing hormone (LH-RH)], a GnRH agonist. The chemical name is 5- oxo-L-prolyl-L-histidyl-L-tryptophyl-L-seryl-L-tyrosyl-D-leucyl-L-leucyl-L-arginyl-N-ethyl-L-prolinamide acetate (salt) with the following structural formula:
LUPRON DEPOT 3.75 mg (leuprolide acetate for depot suspension for injection) is available in a prefilled dual-chamber syringe containing sterile lyophilized microspheres powder which, when mixed with diluent, become a suspension intended as an IM injection.
The front chamber of LUPRON DEPOT 3.75 mg prefilled dual-chamber syringe contains leuprolide acetate for depot suspension (3.75 mg), purified gelatin (0.65 mg), DL-lactic and glycolic acids copolymer (33.1 mg) and D-mannitol (6.6 mg). The second chamber of diluent contains carboxymethylcellulose sodium (5 mg), D-mannitol (50 mg), polysorbate 80 (1 mg), water for injection, USP, and glacial acetic acid, USP to control pH.
During the manufacture of LUPRON DEPOT 3.75 mg, acetic acid is lost, leaving the peptide.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Leuprolide acetate is a long-acting GnRH analog. A single monthly injection of LUPRON DEPOT 3.75 mg results in an initial stimulation followed by a prolonged suppression of pituitary gonadotropins. Repeated dosing of LUPRON DEPOT 3.75 mg at monthly intervals results in decreased secretion of gonadal steroid. Consequently, tissues and functions that depend on gonadal steroids for their maintenance become quiescent. This effect is reversible on discontinuation of drug therapy.
Leuprolide acetate is not active when given orally.
12.2 Pharmacodynamics
Administration of LUPRON DEPOT 3.75 mg in therapeutic doses results in suppression of the pituitary-gonadal system. Normal function is usually restored within three months after treatment is discontinued. Therefore, diagnostic tests of pituitary gonadotropic and gonadal functions conducted during treatment and for up to three months after discontinuation of LUPRON DEPOT 3.75 mg may be affected.
12.3 Pharmacokinetics
Absorption
Following a single IM injection of LUPRON DEPOT 3.75 mg in healthy female volunteers, absorption of leuprolide was characterized by an initial increase in plasma concentration, with peak concentration ranging from 4.6 to 10.2 ng/mL at four hours postdosing. However, intact leuprolide and an inactive metabolite could not be distinguished by the assay used in the study. Following the initial rise, leuprolide concentrations started to plateau within two days after dosing and remained relatively stable for about four to five weeks with plasma concentrations of about 0.30 ng/mL.
Distribution
The mean steady-state volume of distribution of leuprolide following intravenous bolus administration to healthy male volunteers was 27 L. In vitro binding to human plasma proteins ranged from 43% to 49%.
Metabolism
Leuprolide acetate is a peptide that is primarily degraded by peptidase. In healthy male volunteers, a 1 mg bolus of leuprolide administered intravenously revealed that the mean systemic clearance was 7.6 L/h, with a terminal elimination half-life of approximately 3 hours based on a two-compartment model.
The major metabolite (M-I, a pentapeptide) plasma concentrations measured in 5 prostate cancer patients reached maximum concentration 2 to 6 hours after dosing and were approximately 6% of the peak parent drug concentration. One week after dosing, mean plasma M-I concentrations were approximately 20% of mean leuprolide concentrations.
Excretion
Following administration of LUPRON DEPOT 3.75 mg to 3 patients, less than 5% of the dose was recovered as parent and M-I metabolite in the urine.
Use in Specific Populations
The pharmacokinetics of LUPRON DEPOT have not been evaluated in patients with hepatic and renal impairment.
Drug Interactions
No pharmacokinetic drug-drug interaction studies have been conducted with LUPRON DEPOT 3.75 mg. However, leuprolide acetate is a peptide that is not degraded by cytochrome P-450 enzymes; hence, drug interactions associated with cytochrome P-450 enzymes would not be expected to occur.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
A two-year carcinogenicity study was conducted in rats and mice. In rats, a dose-related increase of benign pituitary hyperplasia and benign pituitary adenomas was noted at 24 months when the drug was administered subcutaneously at high daily doses (0.6 to 4 mg/kg). There was a significant but not dose-related increase of pancreatic islet-cell adenomas in females and of testicular interstitial cell adenomas in males (highest incidence in the low dose group). In mice, no leuprolide acetate-induced tumors or pituitary abnormalities were observed at a dose as high as 60 mg/kg for two years. Patients have been treated with leuprolide acetate for up to three years with doses as high as 10 mg/day and for two years with doses as high as 20 mg/day without demonstrable pituitary abnormalities.
Mutagenicity studies have been performed with leuprolide acetate using bacterial and mammalian systems. These studies provided no evidence of a mutagenic potential.
-
14 CLINICAL STUDIES
The safety and efficacy of LUPRON DEPOT 3.75 mg for the indicated populations has been established based on adequate and well-controlled studies in adults (See Table 8) of LUPRON DEPOT 3.75 mg [see Indications and Usage (1)].
14.1 Endometriosis
LUPRON DEPOT 3.75 mg Monotherapy
In controlled clinical studies, LUPRON DEPOT 3.75 mg monthly for six months was shown to be comparable to danazol 800 mg/day in relieving the clinical sign/symptoms of endometriosis (pelvic pain, dysmenorrhea, dyspareunia, pelvic tenderness, and induration) and in reducing the size of endometrial implants as evidenced by laparoscopy.
The clinical significance of a decrease in endometriotic lesions is not known, and laparoscopic staging of endometriosis does not necessarily correlate with the severity of symptoms.
LUPRON DEPOT 3.75 mg monthly induced amenorrhea in 74% and 98% of the women after the first and second month of treatment, respectively. Most of the remaining women reported episodes of only light bleeding or spotting. In the first, second and third post-treatment months, normal menstrual cycles resumed in 7%, 71% and 95% of women, respectively, excluding those who became pregnant.
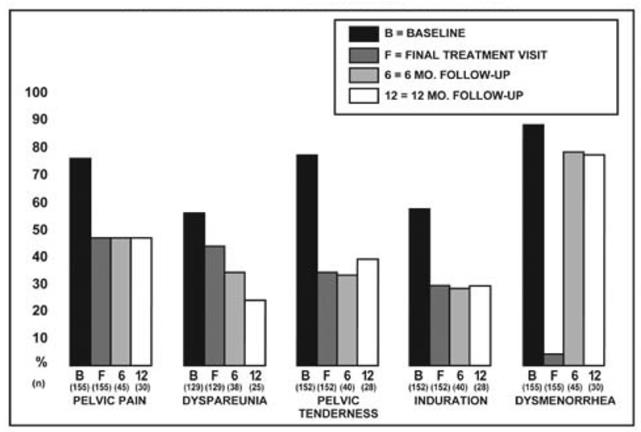
Figure 1 illustrates the percent of women with symptoms at baseline, final treatment visit and sustained relief at 6 and 12 months following discontinuation of treatment for the various symptoms evaluated during the two controlled clinical studies. A total of 166 women received LUPRON DEPOT 3.75 mg. Seventy-five percent (N=125) of these elected to participate in the follow-up period. Of these women, 36% and 24% are included in the 6-month and 12-month follow-up analysis, respectively. All the women who had a pain evaluation at baseline and at least of one treatment visit are included in the Baseline (B) and final treatment visit (F) analysis.
Figure 1. Percent of Women with Signs/Symptoms of Endometriosis at Baseline, Final Treatment Visit, and After 6 and 12 Months of Follow-Up, LUPRON DEPOT 3.75 mg Monthly for Six Months
LUPRON DEPOT with Norethindrone Acetate Add-Back Therapy
Two clinical studies with treatment duration of 12 months were conducted to evaluate the effect of co-administration of LUPRON DEPOT 3.75 mg and norethindrone acetate on the loss of bone mineral density (BMD) associated with LUPRON DEPOT 3.75 mg and on the efficacy of LUPRON DEPOT in relieving symptoms of endometriosis. All women in these studies received calcium supplementation with 1000 mg elemental calcium. A total of 242 women were treated with monthly administration of LUPRON DEPOT 3.75 mg (13 injections) and 191 of them were co-administered 5 mg norethindrone acetate taken daily. The population age range was 17-43 years old. The majority of women were Caucasian (87%).
One co-administration study was a controlled, randomized and double-blind study included 51 women treated monthly with LUPRON DEPOT 3.75 mg alone and 55 women treated monthly with LUPRON DEPOT 3.75 mg plus norethindrone acetate daily. Women in this trial were followed for up to 24 months after completing one year of treatment. The other study was an open-label single arm clinical study in 136 women of one year of treatment with LUPRON DEPOT 3.75 mg monthly and daily norethindrone acetate 5 mg, with follow-up for up to 12 months after completing treatment. See Table 8.
The assessment of efficacy was based on the investigator’s or the woman’s monthly assessment of five signs or symptoms of endometriosis (dysmenorrhea, pelvic pain, deep dyspareunia, pelvic tenderness and pelvic induration).
Table 8 below provides detailed efficacy data regarding relief of symptoms of endometriosis based on the two studies of co-administration of LUPRON DEPOT 3.75 mg monthly and norethindrone acetate 5 mg daily.
Table 8. Effect of LUPRON DEPOT and Norethindrone Acetate on the Symptoms of Endometriosis and Mean Clinical Severity Scores Percent of Women
with SymptomsClinical Pain
Severity ScoreBaseline Final Baseline Final Variable Study Group N1 (%)2 (%) N1 Value3 Change Dysmenorrhea Controlled Study LD*4 51 (100) (4) 50 3.2 -2.0 LD/N† 55 (100) (4) 54 3.1 -2.0 Open Label Study LD/N5 136 (99) (9) 134 3.3 -2.1 Pelvic Pain Controlled Study LD4 51 (100) (66) 50 2.9 -1.1 LD/N 55 (96) (56) 54 3.1 -1.1 Open Label Study LD/N5 136 (99) (63) 134 3.2 -1.2 Deep Dyspareunia Controlled Study LD4 42 (83) (37) 25 2.4 -1.0 LD/N 43 (84) (45) 30 2.7 -0.8 Open Label Study LD/N5 102 (91) (53) 94 2.7 -1.0 Pelvic Tenderness Controlled Study LD4 51 (94) (34) 50 2.5 -1.0 LD/N 54 (91) (34) 52 2.6 -0.9 Open Label Study LD/N5 136 (99) (39) 134 2.9 -1.4 Pelvic Induration Controlled Study LD4 51 (51) (12) 50 1.9 -0.4 LD/N 54 (46) (17) 52 1.6 -0.4 Open Label Study LD/N5 136 (75) (21) 134 2.2 -0.9 * LD = LUPRON DEPOT 3.75 mg assessment
† LD/N = LUPRON DEPOT 3.75 mg plus norethindrone acetate 5 mg
1 Number of women that were included in the assessment
2 Percentage of women with the symptom/sign
3 Value description: 1=none; 2= mild; 3= moderate; 4= severe
4 12-month treatment followed by up to 24 months of follow up
5 12-month treatment followed by up to 12 months of follow upSuppression of menses (menses was defined as three or more consecutive days of menstrual bleeding) was maintained throughout treatment in 84% and 73% of women receiving leuprolide acetate and norethindrone acetate, in the controlled study and open label study, respectively. The median time for menses resumption after treatment with leuprolide acetate and norethindrone acetate was 8 weeks.
Changes in Bone Density
The effect of LUPRON DEPOT 3.75 mg and norethindrone acetate on bone mineral density was evaluated by dual energy x-ray absorptiometry (DEXA) scan in the two clinical trials. For the open-label study, success in mitigating BMD loss was defined as the lower bound of the 95% confidence interval around the change from baseline at one year of treatment not to exceed -2.2%. The bone mineral density data of the lumbar spine from these two studies are presented in Table 9.
Table 9. Mean Percent Change from Baseline in Bone Mineral Density of Lumbar Spine LUPRON DEPOT
3.75 mg (LD only)LUPRON DEPOT 3.75 mg
plus norethindrone acetate 5 mg daily (LD/N)Controlled Study Controlled Study Open Label Study N Change
Mean (95% CI)#N Change
Mean (95% CI)#N Change
Mean (95% CI)#Week 24* 41 -3.2% (-3.8, -2.6) 42 -0.3% (-0.8, 0.3) 115 -0.2% (-0.6, 0.2) Week 52† 29 -6.3% (-7.1, -5.4) 32 -1.0% (-1.9, -0.1) 84 -1.1% (-1.6, -0.5) * Includes on-treatment measurements that fell within 2 to 252 days after the first day of treatment.
† Includes on-treatment measurements >252 days after the first day of treatment.
# 95% CI: 95% Confidence IntervalThe change in BMD following discontinuation of treatment is shown in Table 10.
Table 10. Mean Percent Change from Baseline in BMD of Lumbar Spine in Post-Treatment Follow-up Period1 Post Treatment
MeasurementControlled Study Open Label Study LD-Only LD/N LD/N N Mean
%
Change95% CI
(%)2N Mean
%
Change95% CI
(%)N Mean
%
Change95% CI
(%)2Month 8 19 -3.3 (-4.9, -1.8) 23 -0.9 (-2.1, 0.4) 89 -0.6 (-1.2, 0.0) Month 12 16 -2.2 (-3.3, -1.1) 12 -0.7 (-2.1, 0.6) 65 0.1 (-0.6, 0.7) 1 Patients with post treatment measurements
2 95% CI (2-sided) of percent change in BMD values from baselineThese clinical studies demonstrated that co-administration of leuprolide acetate and norethindrone acetate 5 mg daily is effective in significantly reducing the loss of bone mineral density that occurs with LUPRON DEPOT 3.75 mg and in relieving symptoms of endometriosis.
14.2 Fibroids
LUPRON DEPOT 3.75 mg monthly for a period of three to six months was studied in four controlled clinical trials.
In one of these clinical studies, enrollment was based on hematocrit ≤ 30% and/or hemoglobin ≤ 10.2 g/dL. Administration of LUPRON DEPOT 3.75 mg monthly, concomitantly with iron, produced an increase of ≥ 6% hematocrit and ≥ 2 g/dL hemoglobin in 77% of women at three months of therapy. The mean change in hematocrit was 10.1% and the mean change in hemoglobin was 4.2 g/dL. Clinical response was judged to be a hematocrit of ≥ 36% and hemoglobin of ≥ 12 g/dL, thus allowing for autologous blood donation prior to surgery. At two and three months, respectively, 71% and 75% of women met this criterion (Table 11). These data suggest however, that some women may benefit from iron alone or 1 to 2 months of LUPRON DEPOT 3.75 mg.
Table 11. Percent of Women Achieving Hematocrit ≥ 36% and Hemoglobin ≥ 12 g/dL Treatment Group Week 4 Week 8 Week 12 LUPRON DEPOT 3.75 mg with Iron (N=104) 40* 71† 75* Iron Alone (N=98) 17 39 49 * P-Value < 0.01
† P-Value < 0.001Excessive vaginal bleeding (menorrhagia or menometrorrhagia) decreased in 80% of women at three months. Episodes of spotting and menstrual-like bleeding were noted in 16% of women at final visit.
In this same study, a decrease in uterine volume and myoma volume of ≥25% was seen in 60% and 54% of women, respectively. The mean fibroid diameter was 6.3 cm at pretreatment and decreased to 5.6 cm at the end of treatment. LUPRON DEPOT 3.75 mg was found to relieve symptoms of bloating, pelvic pain, and pressure.
In three other controlled clinical trials, enrollment was not based on hematologic status. Mean uterine volume decreased by 41% and myoma volume decreased by 37% at final visit as evidenced by ultrasound or MRI. The mean fibroid diameter was 5.6 cm at pretreatment and decreased to 4.7 cm at the end of treatment. These women also experienced a decrease in symptoms including excessive vaginal bleeding and pelvic discomfort. Ninety-five percent of these women became amenorrheic with 61%, 25%, and 4% experiencing amenorrhea during the first, second, and third treatment months respectively.
In addition, post-treatment follow-up was carried out in one clinical trial for a small percentage of women on LUPRON DEPOT 3.75 mg (N=46) among the 77% who demonstrated a ≥ 25% decrease in uterine volume while on therapy. Menses usually returned within two months of cessation of therapy. Mean time to return to pretreatment uterine size was 8.3 months. Regrowth did not appear to be related to pretreatment uterine volume.
Changes in Bone Density
In one of the studies for fibroids described above, when LUPRON DEPOT 3.75 mg was administered for three months in women with uterine fibroids, vertebral trabecular bone mineral density, as assessed by quantitative digital radiography (QDR), revealed a mean decrease of 2.7% compared with baseline. Six months after discontinuation of therapy, a trend toward recovery was observed.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Each LUPRON DEPOT 3.75 mg kit (NDC 0074-3641-03) contains:
- one prefilled dual-chamber syringe
- one plunger
- two alcohol swabs
Each single-dose dual chamber syringe contains sterile white lyophilized microsphere powder of 3.75 mg of leuprolide acetate incorporated in a biodegradable polymer in one chamber and a colorless diluent (1 mL) in the other chamber. When mixed with the diluent, LUPRON DEPOT 3.75 mg for injection, is administered as a single IM injection.
Store between 20° to 25°C (68° to 77°F). Excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
- one prefilled dual-chamber syringe
-
17 PATIENT COUNSELING INFORMATION
Loss of Bone Density
Advise patients about the risk of loss of bone mineral density and that treatment is limited [see Dosage and Administration (2.1)]. Advise patients about other factors that can increase and decrease their risk of bone mineral density loss [see Warnings and Precautions (5.1)].
Embryo-Fetal Toxicity
- Advise females of reproductive potential of the possible risk to a fetus. Advise patients to inform healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.2) and Use in Special Populations (8.1)].
- If contraception is indicated, advise females of reproductive potential to use non-hormonal contraception during treatment with LUPRON DEPOT 3.75 mg [see Use in Special Populations (8.3)].
Hypersensitivity Reactions
Acute Hypersensitivity
Inform patients that acute hypersensitivity reactions, including anaphylaxis, have been reported with LUPRON DEPOT. Advise patients to seek appropriate medical care if symptoms of hypersensitivity reactions occur [see Warnings and Precautions (5.3) and Adverse Reactions (6.2)].
Delayed Hypersensitivity
Inform patients that delayed hypersensitivity reactions including the severe cutaneous adverse reactions (SCAR) of Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) have been very rarely reported in association with leuprolide-containing therapy [see Warnings and Precautions (5.3)]. Advise patients to contact their physician or seek emergency help at the first signs or symptoms of delayed hypersensitivity reactions.
Initial Flare of Symptoms
Advise patients that they may experience an increase in symptoms during the initial days of therapy. Advise patients that these symptoms should dissipate with continued therapy [see Warnings and Precautions (5.4)].
Convulsions
Inform patients that convulsions have been reported in patients who have received LUPRON DEPOT. Advise patients to seek medical attention in the event of a convulsion [see Warnings and Precautions (5.5)].
Clinical Depression
Inform patients that depression may occur or worsen during treatment with GnRH agonists, including LUPRON DEPOT 3.75 mg, especially in patients with a history of depression. Advise patients to immediately report thoughts and behaviors of concern to healthcare providers [see Warnings and Precautions (5.6)].
Manufactured for
AbbVie Inc.
North Chicago, IL 60064
by Takeda Pharmaceutical Company Limited
Osaka, Japan 540-864520082650
- Advise females of reproductive potential of the possible risk to a fetus. Advise patients to inform healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.2) and Use in Special Populations (8.1)].
-
PRINCIPAL DISPLAY PANEL
NDC 0074-3641-03
FOR ADULT USE 3.75 mg for 1 - Month administration
Single Dose Administration Kit with prefilled dual-chamber syringe.
LupronDepot®
(leuprolide acetate for depot suspension)
3.75 mg for 1 - Month administration
FOR INTRAMUSCULAR INJECTION
The front chamber contains: leuprolide acetate 3.75 mg, purified gelatin 0.65 mg, DL-lactic & glycolic acids copolymer 33.1 mg, D-mannitol 6.6 mg
The second chamber contains: D-mannitol 50 mg, carboxymethylcellulose sodium 5 mg, polysorbate 80 1 mg, water for injection, USP, and glacial acetic acid, USP to control pH
Rx only

-
INGREDIENTS AND APPEARANCE
LUPRON DEPOT
leuprolide acetate kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0074-3641 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0074-3641-03 1 in 1 CARTON; Type 0: Not a Combination Product 10/22/1990 2 NDC:0074-3641-71 1 in 1 CARTON; Type 0: Not a Combination Product 10/22/1990 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 SYRINGE 1 mL Part 2 2 PACKET 2 mL Part 1 of 2 LUPRON DEPOT
leuprolide acetate injection, powder, lyophilized, for suspensionProduct Information Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEUPROLIDE ACETATE (UNII: 37JNS02E7V) (LEUPROLIDE - UNII:EFY6W0M8TG) LEUPROLIDE ACETATE 3.75 mg in 1 mL Inactive Ingredients Ingredient Name Strength ACETIC ACID (UNII: Q40Q9N063P) WATER (UNII: 059QF0KO0R) POLYSORBATE 80 (UNII: 6OZP39ZG8H) 1 mg in 1 mL MANNITOL (UNII: 3OWL53L36A) 56.6 mg in 1 mL GELATIN, UNSPECIFIED (UNII: 2G86QN327L) 0.65 mg in 1 mL CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) 5 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020011 10/22/1990 Part 2 of 2 ALCOHOL
isopropyl alcohol swabProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 0.7 mL in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 mL in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 01/20/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020011 10/22/1990 Labeler - AbbVie Inc. (078458370)