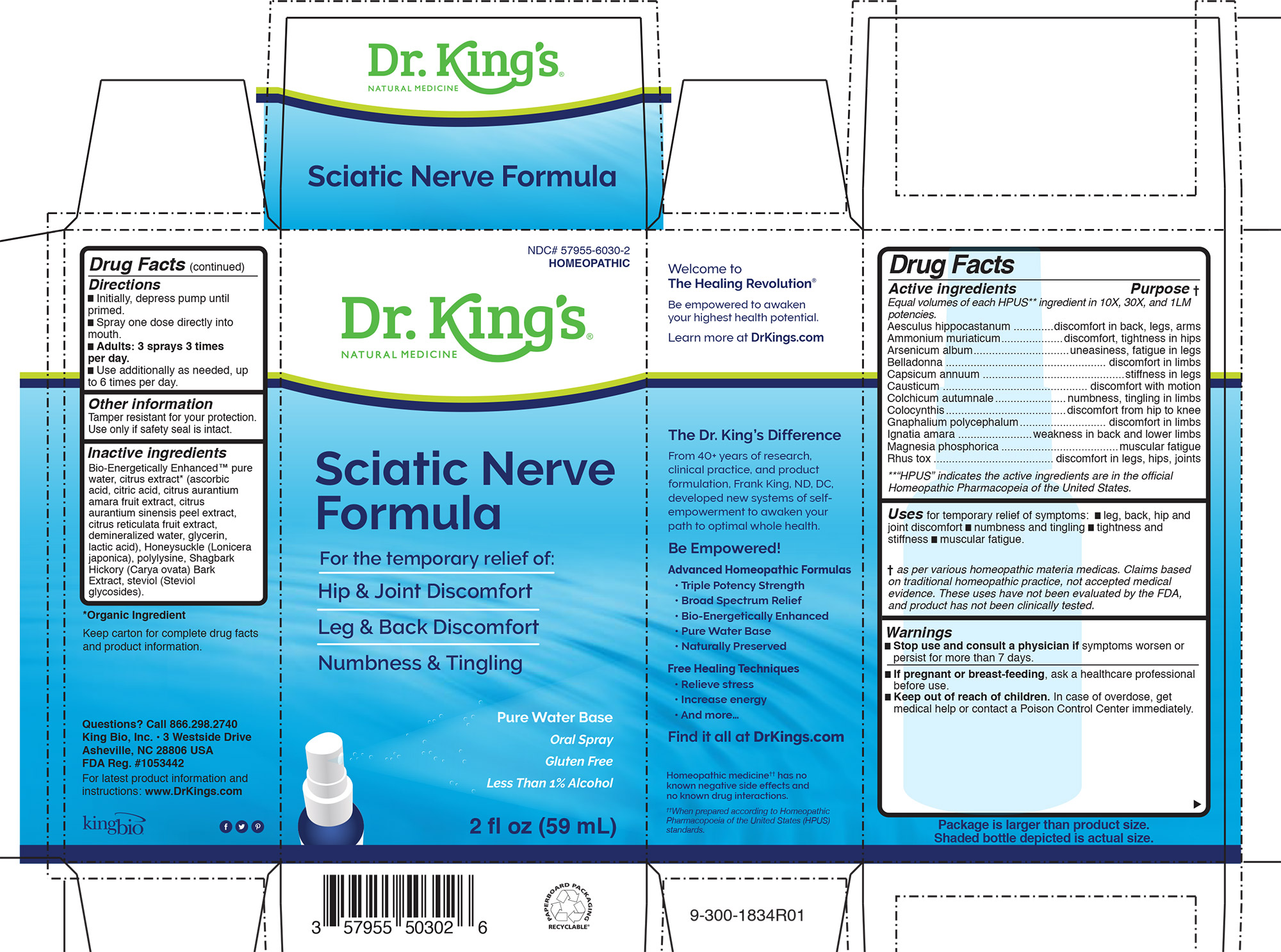
Label: SCIATIC NERVE FORMULA- aesculus hippocastanum, ammonium muriaticum, arsenicum album, belladonna, capsicum annuum, causticum, colchicum autumnale, colocynthis, gnaphalium polycephalum, ignatia amara, magnesia phosphoria, rhus toxicodendron liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 57955-6030-2 - Packager: King Bio Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated August 13, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
Drug Facts
____________________________________________________________________________________________________________
Equal volumes of each ingredient in 10X, 30X, and LM1 potencies.
HPUS active ingredients: Aesculus hippocastanum, Ammonium muriaticum, Arsenicum album, Belladonna, Capsicum annuum, Causticum, Colchicum autumnale, Colocynthis, Gnaphalium polycephalum, Ignatia amara, Magnesia phosphoria, Rhus toxicodendron .
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
-
INACTIVE INGREDIENT
Inactive Ingredients
Bio-Energetically Enhanced™ pure
water, citrus extract* (ascorbic
acid, citric acid, citrus aurantium
amara fruit extract, citrus
aurantium sinensis peel extract,
citrus reticulata fruit extract,
demineralized water, glycerin,
lactic acid), Honeysuckle (Lonicera
japonica), polylysine, Shagbark
Hickory (Carya ovata) Bark
Extract, steviol (Steviol
glycosides). -
PURPOSE
Drug Facts
________________________________________________
Active ingredients Purpose
Equal volumes of each HPUS** ingredient in 10X, 30X, and 1LM
potencies.
Aesculus hippocastanum .............discomfort in back, legs, arms
Ammonium muriaticum....................discomfort, tightness in hips
Arsenicum album...............................uneasiness, fatigue in legs
Belladonna .................................................... discomfort in limbs
Capsicum annuum ..............................................stiffness in legs
Causticum ............................................... discomfort with motion
Colchicum autumnale.......................numbness, tingling in limbs
Colocynthis.......................................discomfort from hip to knee
Gnaphalium polycephalum............................ discomfort in limbs
Ignatia amara ........................weakness in back and lower limbs
Magnesia phosphorica ......................................muscular fatigue
Rhus tox ....................................... discomfort in legs, hips, joints
**“HPUS” indicates the active ingredients are in the official
Homeopathic Pharmacopeia of the United States.
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SCIATIC NERVE FORMULA
aesculus hippocastanum, ammonium muriaticum, arsenicum album, belladonna, capsicum annuum, causticum, colchicum autumnale, colocynthis, gnaphalium polycephalum, ignatia amara, magnesia phosphoria, rhus toxicodendron liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:57955-6030 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HORSE CHESTNUT (UNII: 3C18L6RJAZ) (HORSE CHESTNUT - UNII:3C18L6RJAZ) HORSE CHESTNUT 10 [hp_X] in 59 mL AMMONIUM CHLORIDE (UNII: 01Q9PC255D) (AMMONIUM CATION - UNII:54S68520I4) AMMONIUM CATION 10 [hp_X] in 59 mL ARSENIC TRIOXIDE (UNII: S7V92P67HO) (ARSENIC CATION (3+) - UNII:C96613F5AV) ARSENIC TRIOXIDE 10 [hp_X] in 59 mL ATROPA BELLADONNA (UNII: WQZ3G9PF0H) (ATROPA BELLADONNA - UNII:WQZ3G9PF0H) ATROPA BELLADONNA 10 [hp_X] in 59 mL CAPSICUM (UNII: 00UK7646FG) (CAPSICUM - UNII:00UK7646FG) CAPSICUM 10 [hp_X] in 59 mL CAUSTICUM (UNII: DD5FO1WKFU) (CAUSTICUM - UNII:DD5FO1WKFU) CAUSTICUM 10 [hp_X] in 59 mL COLCHICUM AUTUMNALE BULB (UNII: 993QHL78E6) (COLCHICUM AUTUMNALE BULB - UNII:993QHL78E6) COLCHICUM AUTUMNALE BULB 10 [hp_X] in 59 mL CITRULLUS COLOCYNTHIS FRUIT PULP (UNII: 23H32AOH17) (CITRULLUS COLOCYNTHIS FRUIT PULP - UNII:23H32AOH17) CITRULLUS COLOCYNTHIS FRUIT PULP 10 [hp_X] in 59 mL PSEUDOGNAPHALIUM OBTUSIFOLIUM (UNII: 36XQ854NWW) (PSEUDOGNAPHALIUM OBTUSIFOLIUM - UNII:36XQ854NWW) PSEUDOGNAPHALIUM OBTUSIFOLIUM 10 [hp_X] in 59 mL STRYCHNOS IGNATII SEED (UNII: 1NM3M2487K) (STRYCHNOS IGNATII SEED - UNII:1NM3M2487K) STRYCHNOS IGNATII SEED 10 [hp_X] in 59 mL MAGNESIUM PHOSPHATE, DIBASIC TRIHYDRATE (UNII: HF539G9L3Q) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM PHOSPHATE, DIBASIC TRIHYDRATE 10 [hp_X] in 59 mL TOXICODENDRON PUBESCENS LEAF (UNII: 6IO182RP7A) (TOXICODENDRON PUBESCENS LEAF - UNII:6IO182RP7A) TOXICODENDRON PUBESCENS LEAF 10 [hp_X] in 59 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CARYA OVATA BARK (UNII: X765CF609L) CITRUS BIOFLAVONOIDS (UNII: BD70459I50) LONICERA JAPONICA FLOWER (UNII: 4465L2WS4Y) POLYEPSILON-LYSINE (4000 MW) (UNII: WB0M8X4TWR) REBAUDIOSIDE A (UNII: B3FUD0528F) Product Characteristics Color Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57955-6030-2 1 in 1 CARTON 02/01/2019 1 59 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 02/01/2019 Labeler - King Bio Inc. (617901350) Registrant - King Bio Inc. (617901350) Establishment Name Address ID/FEI Business Operations King Bio Inc. 617901350 api manufacture(57955-6030) , manufacture(57955-6030)