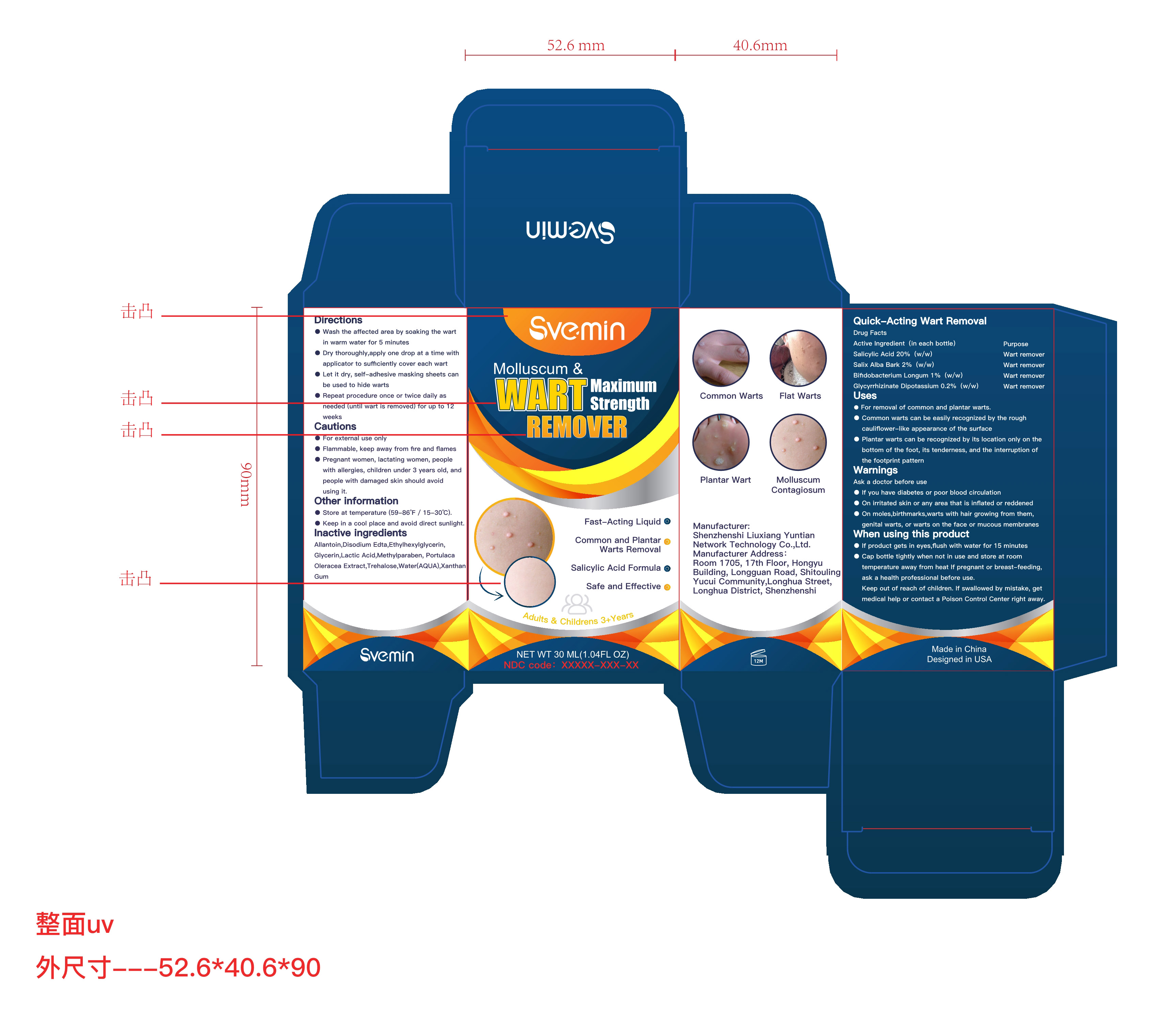
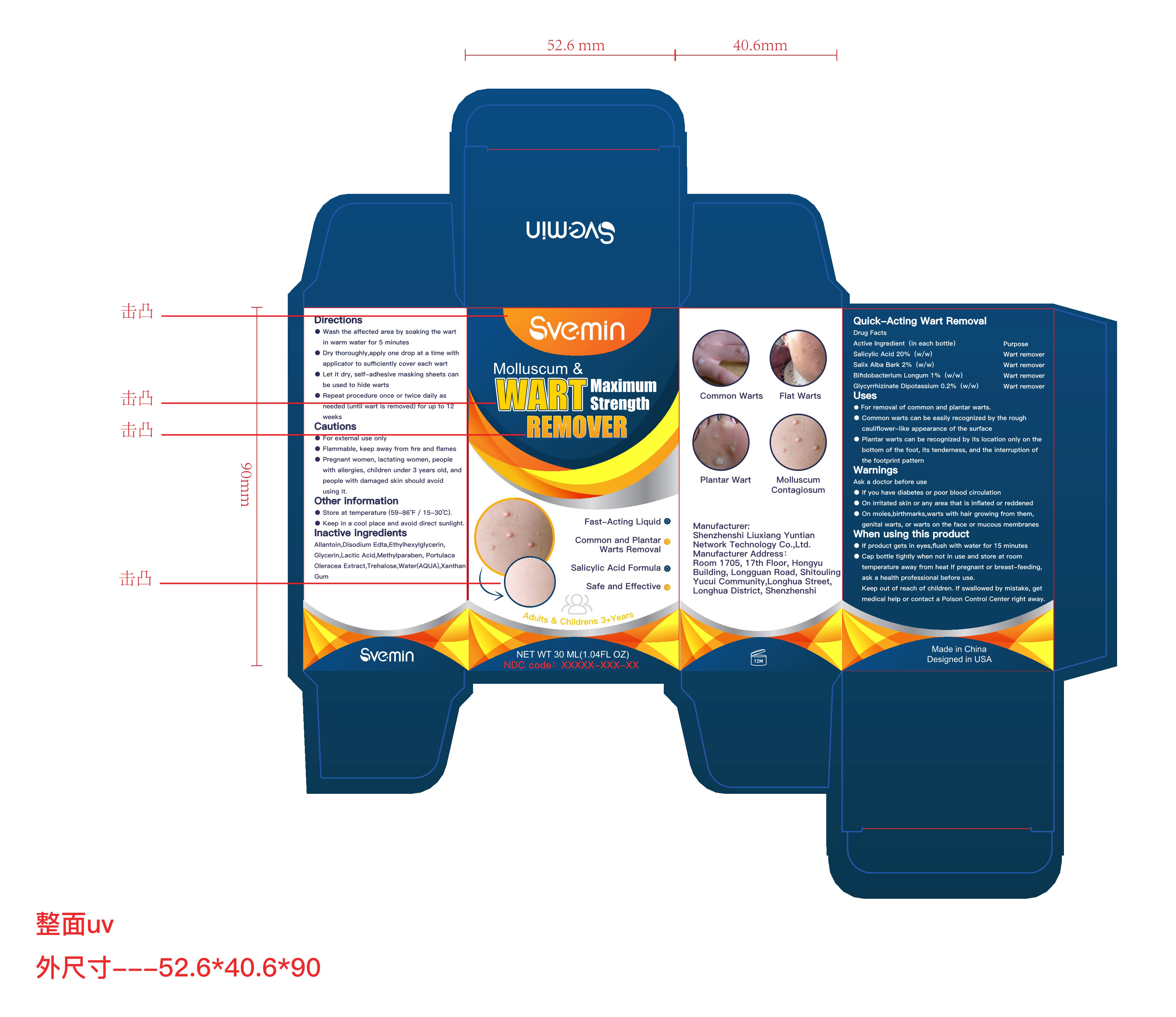
Label: QUICK-ACTING WART REMOVAL liquid
- NDC Code(s): 83575-001-01
- Packager: Shenzhenshi Liuxiang Yuntian Network Technology Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 11, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- Purpose
- Uses
- Warnings
- DO NOT USE
-
When using this product
- If product gets in eyes,flush with water for 15 minutes
- Cap bottle tightly when not in use and store at room temperature away from heat If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. If swallowed by mistake, get medical help or contact a Poison Control Center right away.
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
Directions
-Wash the affffected area by soaking the wart in warm water for 5 minutes
- Dry thoroughly,apply one drop at a time with applicator to suffiffifficiently cover each wart
- Let it dry, self-adhesive masking sheets can be used to hide warts
- Repeat procedure once or twice daily as needed (until wart is removed) for up to 12 weeks
- Other information
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
QUICK-ACTING WART REMOVAL
quick-acting wart removal liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83575-001 Route of Administration CUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLYCYRRHIZINATE DIPOTASSIUM (UNII: CA2Y0FE3FX) (GLYCYRRHIZIN - UNII:6FO62043WK) GLYCYRRHIZINATE DIPOTASSIUM 0.2 mg in 100 mL SALIX ALBA BARK (UNII: 205MXS71H7) (SALIX ALBA BARK - UNII:205MXS71H7) SALIX ALBA BARK 2 mg in 100 mL SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 20 mg in 100 mL BIFIDOBACTERIUM LONGUM (UNII: 831AQW699W) (BIFIDOBACTERIUM LONGUM - UNII:831AQW699W) BIFIDOBACTERIUM LONGUM 1 mg in 100 mL Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) GLYCERIN (UNII: PDC6A3C0OX) XANTHAN GUM (UNII: TTV12P4NEE) LACTIC ACID (UNII: 33X04XA5AT) PURSLANE (UNII: M6S840WXG5) WATER (UNII: 059QF0KO0R) ALLANTOIN (UNII: 344S277G0Z) METHYLPARABEN (UNII: A2I8C7HI9T) TREHALOSE (UNII: B8WCK70T7I) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83575-001-01 30 mL in 1 BOX; Type 0: Not a Combination Product 07/12/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M016 07/12/2023 Labeler - Shenzhenshi Liuxiang Yuntian Network Technology Co., Ltd. (713044573) Establishment Name Address ID/FEI Business Operations Shenzhenshi Liuxiang Yuntian Network Technology Co., Ltd. 713044573 manufacture(83575-001)