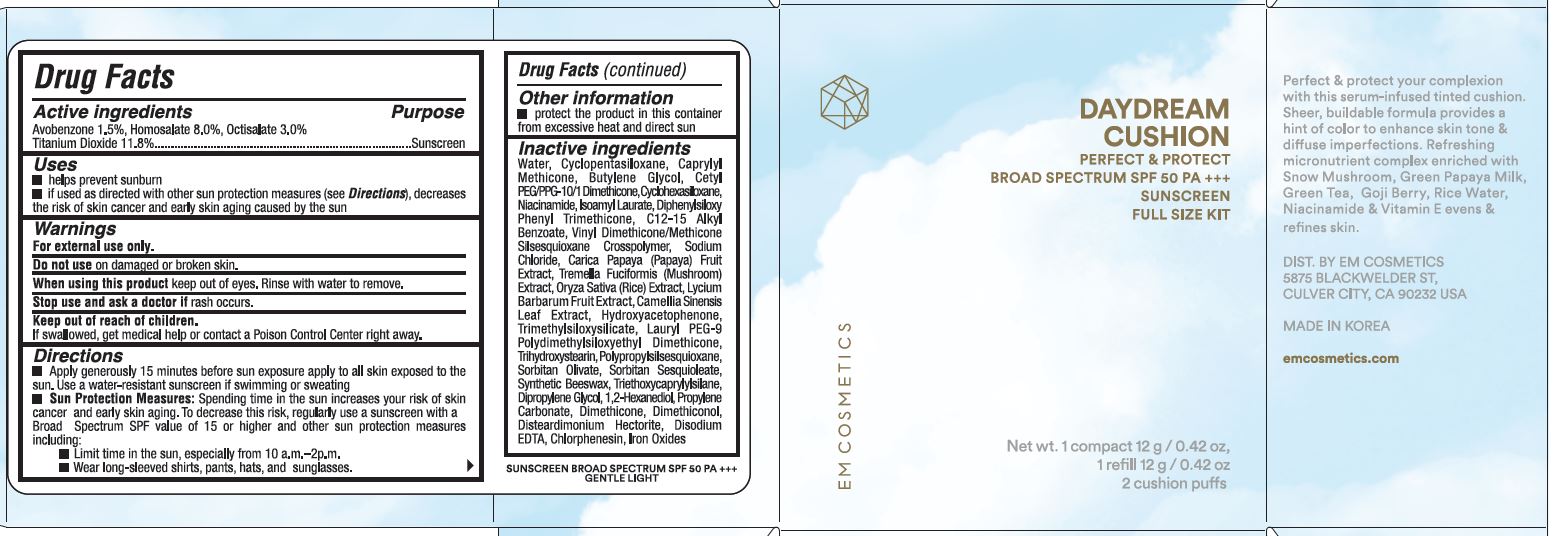
Label: DAYDREAM CUSHION GENTLE LIGHT- avobenzone, homosalate, octisalate, titanium dioxide cream
- NDC Code(s): 73586-103-11
- Packager: EM Cosmetics LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 29, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENTS
- Purpose
- USES
- WARNINGS
-
DIRECTIONS
- Apply generously 15 minutes before sun exposure apply to all skin exposed to the sun. Use a water-resistant sunscreen if swimming or sweating.
- Sun Protection Measures: Spending time in the sun increases your risk of skin cancer & early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses
- OTHER INFORMATION
-
INACTIVE INGREDIENTS
WATER, CYCLOPENTASILOXANE, CAPRYLYL METHICONE, BUTYLENE GLYCOL, CETYL PEG/PPG-10/1 DIMETHICONE, CYCLOHEXASILOXANE, NIACINAMIDE, ISOAMYL LAURATE, DIPHENYLSILOXY PHENYL TRIMETHICONE, C12-15 ALKYL BENZOATE, VINYL DIMETHICONE/METHICONE SILSEQUIOXANE CROSSPOLYMER, SODIUM CHLORIDE, CARICA PAPAYA (PAPAYA) FRUIT EXTRACT, TREMELLA FUCIFORMIS (MUSHROOM) EXTRACT, ORYZA SATIVA (RICE) EXTRACT, LYCIUM BARBARUM FRUIT EXTRACT, CAMELIA SINENSIS LEAF EXTRACT, HYDROXYACETOPHENONE, TRIMETHYLSILOXYSILICATE, LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE, TRIHYDROXYSTEARIN, POLYPROPYLSILSESQUIOXANE, SORBITAN OLIVATE, SORBITAN SESQUIOLATE, SYNTHETIC BEESWAX, TRIETHOXYCAPRYLYLSILANE, DIPROPYLENE GLYCOL, 1,2-HEXANEDIOL, PROPYLENE CARBONATE, DIMETHICONE, DIMETHICONAL, DISTEARDIMONIUM HECTORITE, DISODIUM EDTA, CHLORPHENESIN, IRON OXIDES
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DAYDREAM CUSHION GENTLE LIGHT
avobenzone, homosalate, octisalate, titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73586-103 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 1.5 g in 100 g HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 8 g in 100 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 3 g in 100 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 11.8 g in 100 g Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) WATER (UNII: 059QF0KO0R) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 1.5) (UNII: V2W71V8T0X) CYCLOMETHICONE 6 (UNII: XHK3U310BA) NIACINAMIDE (UNII: 25X51I8RD4) ISOAMYL LAURATE (UNII: M1SLX00M3M) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) VINYL DIMETHICONE/METHICONE SILSESQUIOXANE CROSSPOLYMER (UNII: 9NH1UDD2RR) SODIUM CHLORIDE (UNII: 451W47IQ8X) PAPAYA (UNII: KU94FIY6JB) TREMELLA FUCIFORMIS WHOLE (UNII: 4938BNS0GU) WHITE RICE (UNII: A195V20H7A) LYCIUM BARBARUM FRUIT (UNII: 930626MWDL) GREEN TEA LEAF (UNII: W2ZU1RY8B0) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) TRIMETHYLSILOXYSILICATE (M/Q 1.0-1.2) (UNII: 78GX033D7I) LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: 25G622K2RA) TRIHYDROXYSTEARIN (UNII: 06YD7896S3) PROPYLSILSESQUIOXANE, HYDROGEN TERMINATED (UNII: 2PDG9JR76G) SORBITAN OLIVATE (UNII: MDL271E3GR) SORBITAN SESQUIOLEATE (UNII: 0W8RRI5W5A) YELLOW WAX (UNII: 2ZA36H0S2V) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DIPROPYLENE GLYCOL (UNII: E107L85C40) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) PROPYLENE CARBONATE (UNII: 8D08K3S51E) DIMETHICONE (UNII: 92RU3N3Y1O) DIMETHICONOL (2000 CST) (UNII: T74O12AN6Y) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) EDETATE DISODIUM (UNII: 7FLD91C86K) CHLORPHENESIN (UNII: I670DAL4SZ) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73586-103-11 12 g in 1 CONTAINER; Type 0: Not a Combination Product 10/06/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 10/06/2020 Labeler - EM Cosmetics LLC (065677626)