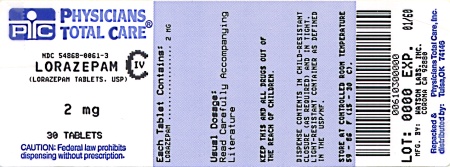
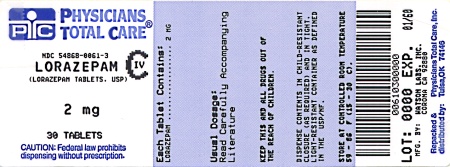
Label: LORAZEPAM tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 54868-0061-2, 54868-0061-3, 54868-0061-4, 54868-0061-5, view more54868-0061-6, 54868-1338-0, 54868-1338-1, 54868-1338-2, 54868-1338-3, 54868-1338-4, 54868-1338-6, 54868-1338-7, 54868-1338-8, 54868-1338-9, 54868-2145-0, 54868-2145-2, 54868-2145-3, 54868-2145-4, 54868-2145-5, 54868-2145-6, 54868-2145-9 - Packager: Physicians Total Care, Inc.
- This is a repackaged label.
- Source NDC Code(s): 0591-0240, 0591-0241, 0591-0242
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIV
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 7, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Lorazepam, an antianxiety agent, has the chemical formula, (±)-7-Chloro-5-(o-chlorophenyl)-1,3-dihydro-3-hydroxy-2H-1,4-benzodiazepin-2-one:
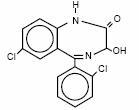
It is a nearly white powder almost insoluble in water. Each lorazepam tablet, to be taken orally, contains 0.5 mg, 1 mg or 2 mg of lorazepam. This product contains the following inactive ingredients: lactose, magnesium stearate, microcrystalline cellulose and polacrilin potassium.
-
CLINICAL PHARMACOLOGY
Studies in healthy volunteers show that in single high doses lorazepam has a tranquilizing action on the central nervous system with no appreciable effect on the respiratory or cardiovascular systems.
Lorazepam is readily absorbed with an absolute bioavailability of 90 percent. Peak concentrations in plasma occur approximately 2 hours following administration. The peak plasma level of lorazepam from a 2 mg dose is approximately 20 ng/ml.
The mean half-life of unconjugated lorazepam in human plasma is about 12 hours and for its major metabolite, lorazepam glucuronide, about 18 hours. At clinically relevant concentrations, lorazepam is approximately 85% bound to plasma proteins. Lorazepam is rapidly conjugated at its 3-hydroxy group into lorazepam glucuronide which is then excreted in the urine. Lorazepam glucuronide has no demonstrable CNS activity in animals.
The plasma levels of lorazepam are proportional to the dose given. There is no evidence of accumulation of lorazepam on administration up to six months.
Studies comparing young and elderly subjects have shown that the pharmacokinetics of lorazepam remain unaltered with advancing age.
-
INDICATIONS AND USAGE
Lorazepam is indicated for the management of anxiety disorders or for the short-term relief of the symptoms of anxiety or anxiety associated with depressive symptoms. Anxiety or tension associated with the stress of everyday life usually does not require treatment with an anxiolytic.
The effectiveness of lorazepam in long-term use, that is, more than 4 months, has not been assessed by systematic clinical studies. The physician should periodically reassess the usefulness of the drug for the individual patient.
- CONTRAINDICATIONS
-
WARNINGS
Lorazepam is not recommended for use in patients with a primary depressive disorder of psychosis. As with all patients on CNS-acting drugs, patients receiving lorazepam should be warned not to operate dangerous machinery or motor vehicles and that their tolerance for alcohol and other CNS depressants will be diminished.
-
PRECAUTIONS
In patients with depression accompanying anxiety, a possibility for suicide should be borne in mind.
For elderly or debilitated patients, the initial daily dosage should not exceed 2 mg in order to avoid oversedation.
Lorazepam dosage should be terminated gradually, since abrupt withdrawal of any antianxiety agent may result in symptoms similar to those for which patients are being treated: anxiety, agitation, irritability, tension, insomnia, and occasional convulsions.
The usual precautions for treating patients with impaired renal or hepatic function should be observed.
In patients where gastrointestinal or cardiovascular disorders coexist with anxiety, it should be noted that lorazepam has not been shown to be of significant benefit in treating the gastrointestinal or cardiovascular component.
Esophageal dilation occurred in rats treated with lorazepam for more than one year at 6 mg/kg/day. The no-effect dose was 1.25 mg/kg/day (approximately 6 times the maximum human therapeutic dose of 10 mg per day). The effect was reversible only when the treatment was withdrawn within two months of first observation of the phenomenon. The clinical significance of this is unknown. However, use of lorazepam for prolonged periods and in geriatric patients requires caution and there should be frequent monitoring for symptoms of upper G.l. disease.
Safety and effectiveness of lorazepam in children of less than 12 years have not been established.
INFORMATION FOR PATIENTSTo assure the safe and effective use of lorazepam, patients should be informed that, since benzodiazepines may produce psychological and physical dependence, it is advisable that they consult with their physician before either increasing the dose or abruptly discontinuing this drug.
ESSENTIAL LABORATORY TESTSSome patients on lorazepam have developed leukopenia, and some have had elevations of LDH. As with other benzodiazepines, periodic blood counts and liver-function tests are recommended for patients on long-term therapy.
CLINICALLY SIGNIFICANT DRUG INTERACTIONSThe benzodiazepines, including lorazepam, produce CNS-depressant effects when administered with such medications as barbiturates or alcohol.
CARCINOGENESIS AND MUTAGENESISNo evidence of carcinogenic potential emerged in rats during an 18-month study with lorazepam. No studies regarding mutagenesis have been performed.
PREGNANCYReproductive studies in animals were performed in mice, rats, and two strains of rabbits. Occasional anomalies (reduction of tarsals, tibia, metatarsals, malrotated limbs, gastroschisis, malformed skull, and microphthalmia) were seen in drug-treated rabbits without relationship to dosage. Although all of these anomalies were not present in the concurrent control group, they have been reported to occur randomly in historical controls. At doses of 40 mg/kg and higher, there was evidence of fetal resorption and increased fetal loss in rabbits which was not seen at lower doses.
The clinical significance of the above findings is not known. However, an increased risk of congenital malformations associated with the use of minor tranquilizers (chlordiazepoxide, diazepam, and meprobamate) during the first trimester of pregnancy has been suggested in several studies. Because the use of these drugs is rarely a matter of urgency, the use of lorazepam during this period should almost always be avoided. The possibility that a woman of childbearing potential may be pregnant at the time of institution of therapy should be considered. Patients should be advised that if they become pregnant, they should communicate with their physician about the desirability of discontinuing the drug.
In humans, blood levels obtained from umbilical cord blood indicate placental transfer of lorazepam and lorazepam glucuronide.
NURSING MOTHERSIt is not known whether oral lorazepam is excreted in human milk like the other benzodiazepine tranquilizers. As a general rule, nursing should not be undertaken while a patient is on a drug since many drugs are excreted in human milk.
-
ADVERSE REACTIONS
Adverse reactions, if they occur, are usually observed at the beginning of therapy and generally disappear on continued medication or upon decreasing the dose. In a sample of about 3,500 anxious patients, the most frequent adverse reaction to lorazepam is sedation (15.9%), followed by dizziness (6.9%), weakness (4.2%), and unsteadiness (3.4%). Less frequent adverse reactions are disorientation, depression, nausea, change in appetite, headache, sleep disturbance, agitation, dermatological symptoms, eye-function disturbance, together with various gastrointestinal symptoms and autonomic manifestations. The incidence of sedation and unsteadiness increased with age.
Small decreases in blood pressure have been noted but are not clinically significant, probably being related to the relief of anxiety produced by lorazepam.
Transient amnesia or memory impairment has been reported in association with the use of benzodiazepines.
-
DRUG ABUSE AND DEPENDENCE
Withdrawal symptoms, similar in character to those noted with barbiturates and alcohol (convulsions, tremor, abdominal and muscle cramps, vomiting, and sweating), have occurred following abrupt discontinuance of lorazepam. The more severe withdrawal symptoms have usually been limited to those patients who received excessive doses over an extended period of time. Generally milder withdrawal symptoms (e.g., dysphoria and insomnia) have been reported following abrupt discontinuance of benzodiazepines taken continuously at therapeutic levels for several months. Consequently, after extended therapy, abrupt discontinuation should generally be avoided and a gradual dosage-tapering schedule followed. Addiction-prone individuals (such as drug addicts or alcoholics) should be under careful surveillance when receiving lorazepam or other psychotropic agents because of the predisposition of such patients to habituation and dependence.
Lorazepam tablets are classified by the Drug Enforcement Administration as a Schedule IV controlled substance.
-
OVERDOSAGE
In the management of overdosage with any drug, it should be borne in mind that multiple agents may have been taken.
Manifestations of lorazepam overdosage include somnolence, confusion, and coma. Induced vomiting and/or gastric lavage should be undertaken, followed by general supportive care, monitoring of vital signs, and close observation of the patient. Hypotension, though unlikely, usually may be controlled with norepinephrine bitartrate injection. The usefulness of dialysis has not been determined.
Flumazenil, a specific benzodiazepine receptor antagonist, is indicated for the complete or partial reversal of the sedative effects of benzodiazepines and may be used in situations when an overdose with a benzodiazepine is known or suspected. Prior to the administration of flumazenil, necessary measures should be instituted to secure airway, ventilation, and intravenous access. Flumazenil is intended as an adjunct to, not as a substitute for, proper management of benzodiazepine overdose. Patients treated with flumazenil should be monitored for re-sedation, respiratory depression, and other residual benzodiazepine effects for an appropriate period after treatment. The prescriber should be aware of a risk of seizure in association with flumazenil treatment, particularly in long-term benzodiazepine users and in cyclic antidepressant overdose. The complete flumazenil package insert including CONTRAINDICATIONS,WARNINGS, and PRECAUTIONS should be consulted prior to use.
-
DOSAGE AND ADMINISTRATION
Lorazepam is administered orally. For optimal results, dose, frequency of administration, and duration of therapy should be individualized according to patient response. To facilitate this, 0.5 mg, 1 mg, and 2 mg tablets are available.
The usual range is 2 to 6 mg/day given in divided doses, the largest dose being taken before bedtime, but the daily dosage may vary from 1 to 10 mg/day.
For anxiety, most patients require an initial dose of 2 to 3 mg/day given b.i.d. or t.i.d.
For insomnia due to anxiety or transient situational stress, a single daily dose of 2 to 4 mg may be given, usually at bedtime.
For elderly or debilitated patients, an initial dosage of 1 to 2 mg/day in divided doses is recommended, to be adjusted as needed and tolerated.
The dosage of lorazepam should be increased gradually when needed to help avoid adverse effects. When higher dosage is indicated, the evening dose should be increased before the daytime doses.
-
HOW SUPPLIED
Lorazepam tablets are available in the following dosage strengths:
0.5 mg: white, scored, round flat faced beveled edge, debossed with 240 over 0.5 on one side and WATSON on the other side, supplied in:
Bottles of 10
NDC 54868-2145-0
Bottles of 20
NDC 54868-2145-2
Bottles of 30
NDC 54868-2145-3
Bottles of 50
NDC 54868-2145-5
Bottles of 60
NDC 54868-2145-6
Bottles of 90
NDC 54868-2145-9
Bottles of 100
NDC 54868-2145-4
1 mg: white, scored, round flat faced beveled edge, debossed with 241 over 1 on one side and WATSON on the other side, supplied in:
Bottles of 03
NDC 54868-1338-6
Bottles of 10
NDC 54868-1338-7
Bottles of 15
NDC 54868-1338-0
Bottles of 20
NDC 54868-1338-1
Bottles of 30
NDC 54868-1338-3
Bottles of 60
NDC 54868-1338-4
Bottles of 90
NDC 54868-1338-8
Bottles of 100
NDC 54868-1338-2
Bottles of 120
NDC 54868-1338-9
2 mg: white, scored, round flat faced beveled edge, debossed with 242 over 2 on one side and WATSON on the other side, supplied in:
Bottles of 30
NDC 54868-0061-3
Bottles of 60
NDC 54868-0061-5
Bottles of 90
NDC 54868-0061-4
Bottles of 100
NDC 54868-0061-2
Bottles of 120
NDC 54868-0061-6
Store at controlled room temperature 15°-30°C (59°-86°F). [See USP.]
Dispense in a tight, light-resistant container as defined in the USP.
Watson Laboratories, Inc.
Corona, CA 92880 USA30223-3
Rev: February 2004
Repackaging and Relabeling by:
Physicians Total Care, Inc.
Tulsa, OK 74146
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LORAZEPAM
lorazepam tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54868-2145(NDC:0591-0240) Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LORAZEPAM (UNII: O26FZP769L) (LORAZEPAM - UNII:O26FZP769L) LORAZEPAM 0.5 mg Inactive Ingredients Ingredient Name Strength LACTOSE (UNII: J2B2A4N98G) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLACRILIN POTASSIUM (UNII: 0BZ5A00FQU) Product Characteristics Color white Score 2 pieces Shape ROUND (Round) Size 6mm Flavor Imprint Code 240;0;5;WATSON Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54868-2145-0 10 in 1 BOTTLE 2 NDC:54868-2145-2 20 in 1 BOTTLE 3 NDC:54868-2145-3 30 in 1 BOTTLE 4 NDC:54868-2145-4 100 in 1 BOTTLE 5 NDC:54868-2145-5 50 in 1 BOTTLE 6 NDC:54868-2145-6 60 in 1 BOTTLE 7 NDC:54868-2145-9 90 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA072926 06/29/1993 LORAZEPAM
lorazepam tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54868-1338(NDC:0591-0241) Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LORAZEPAM (UNII: O26FZP769L) (LORAZEPAM - UNII:O26FZP769L) LORAZEPAM 1 mg Inactive Ingredients Ingredient Name Strength LACTOSE (UNII: J2B2A4N98G) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLACRILIN POTASSIUM (UNII: 0BZ5A00FQU) Product Characteristics Color white Score 2 pieces Shape ROUND (Round) Size 7mm Flavor Imprint Code 241;1;WATSON Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54868-1338-0 15 in 1 BOTTLE 2 NDC:54868-1338-1 20 in 1 BOTTLE 3 NDC:54868-1338-2 100 in 1 BOTTLE 4 NDC:54868-1338-3 30 in 1 BOTTLE 5 NDC:54868-1338-4 60 in 1 BOTTLE 6 NDC:54868-1338-6 03 in 1 BOTTLE 7 NDC:54868-1338-7 10 in 1 BOTTLE 8 NDC:54868-1338-8 90 in 1 BOTTLE 9 NDC:54868-1338-9 120 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA072927 07/23/1993 LORAZEPAM
lorazepam tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54868-0061(NDC:0591-0242) Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LORAZEPAM (UNII: O26FZP769L) (LORAZEPAM - UNII:O26FZP769L) LORAZEPAM 2 mg Inactive Ingredients Ingredient Name Strength LACTOSE (UNII: J2B2A4N98G) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLACRILIN POTASSIUM (UNII: 0BZ5A00FQU) Product Characteristics Color white Score 2 pieces Shape ROUND (Round) Size 7mm Flavor Imprint Code 242;2;WATSON Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54868-0061-2 100 in 1 BOTTLE 2 NDC:54868-0061-3 30 in 1 BOTTLE 3 NDC:54868-0061-4 90 in 1 BOTTLE 4 NDC:54868-0061-5 60 in 1 BOTTLE 5 NDC:54868-0061-6 120 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA072928 12/20/1995 Labeler - Physicians Total Care, Inc. (194123980) Establishment Name Address ID/FEI Business Operations Physicians Total Care, Inc. 194123980 relabel, repack