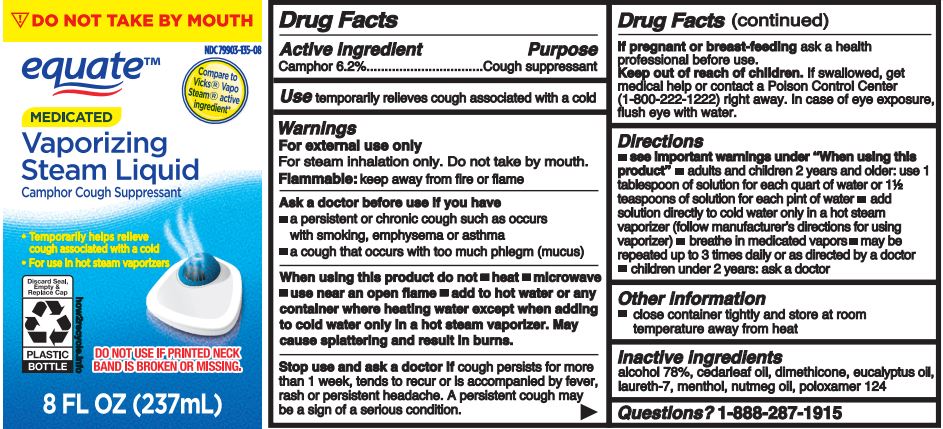
Label: EQUATE- vaporizing steam liquid liquid
- NDC Code(s): 79903-135-08
- Packager: Walmart Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 30, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Use
-
Warnings
For external use only
For steam inhalation only. Do not take by mouth.
Flammable: keep away from fire or flame
Ask a doctor before use if you have
- a persistent or chronic cough such as occurs with smoking, emphysema or asthma
- a cough that occurs with too much phlegm (mucus)
When using this product do not
- heat
- microwave
- use near an open flame
- take by mouth
- add to hot water or any container where heating water except when adding to cold water only in a hot steam vaporizer. May cause splattering and result in burns.
-
Directions
- see important warnings under "When using this product"
- adults & children 2 years & over:
- use 1 tablespoon of solution for each quart of water or 1½ teaspoonful of solution for each pint of water
- add solution directly to cold water only in a hot steam vaporizer (follow manufacturer's directions for using vaporizer)
- breathe in the medicated vapors
- use up to three times daily or as directed by a doctor
- children under 2 years: ask a doctor
- Other information
- Inactive ingredient
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
EQUATE
vaporizing steam liquid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:79903-135 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 6.23 g in 10 mL Inactive Ingredients Ingredient Name Strength DIMETHICONE (UNII: 92RU3N3Y1O) POLOXAMER 124 (UNII: 1S66E28KXA) CEDAR LEAF OIL (UNII: BJ169U4NLG) EUCALYPTUS GLOBULUS LEAF (UNII: S546YLW6E6) LAURETH-7 (UNII: Z95S6G8201) ALCOHOL (UNII: 3K9958V90M) MENTHOL (UNII: L7T10EIP3A) NUTMEG OIL (UNII: Z1CLM48948) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:79903-135-08 237 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 01/01/2023 Labeler - Walmart Inc. (051957769)