Label: CLOPIDOGREL- clopidogrel bisulfate tablet
- NDC Code(s): 50090-7467-0
- Packager: A-S Medication Solutions
- This is a repackaged label.
- Source NDC Code(s): 67877-276
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use CLOPIDOGREL TABLETS safely and effectively. See full prescribing information for CLOPIDOGREL TABLETS. CLOPIDOGREL tablets ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: DIMINISHED ANTIPLATELET EFFECT IN PATIENTS WITH TWO LOSS-OF-FUNCTION ALLELES OF THE CYP2C19 GENE
The effectiveness of clopidogrel tablets results from its antiplatelet activity, which is dependent on its conversion to an active metabolite by the cytochrome P450 (CYP) system, principally CYP2C19 [see Warnings and Precautions (5.1), Clinical Pharmacology (12.3)]. Clopidogrel tablets at recommended doses forms less of the active metabolite and so has a reduced effect on platelet activity in patients who are homozygous for nonfunctional alleles of the CYP2C19 gene, (termed “CYP2C19 poor metabolizers”). Tests are available to identify patients who are CYP2C19 poor metabolizers [see Clinical Pharmacology (12.5)]. Consider use of another platelet P2Y12 inhibitor in patients identified as CYP2C19 poor metabolizers.
Close -
1 INDICATIONS AND USAGE1.1 Acute Coronary Syndrome (ACS) • Clopidogrel tablets is indicated to reduce the rate of myocardial infarction (MI) and stroke in patients with non-ST-segment elevation ACS (unstable angina ...
-
2 DOSAGE AND ADMINISTRATION2.1 Acute Coronary Syndrome - In patients who need an antiplatelet effect within hours, initiate clopidogrel tablets with a single 300 mg oral loading dose and then continue at 75 mg once daily ...
-
3 DOSAGE FORMS AND STRENGTHS• 75 mg tablets: Pink colored, biconvex, round shaped, beveled edge, film coated tablets printed "CL 75" on one side and plain on other side.
-
4 CONTRAINDICATIONS4.1 Active Bleeding - Clopidogrel tablets is contraindicated in patients with active pathological bleeding such as peptic ulcer or intracranial hemorrhage. 4.2 Hypersensitivity - Clopidogrel ...
-
5 WARNINGS AND PRECAUTIONS5.1 Diminished Antiplatelet Activity in Patients with Impaired CYP2C19 Function - Clopidogrel is a prodrug. Inhibition of platelet aggregation by clopidogrel is achieved through an active ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are discussed below and elsewhere in the labeling: • Bleeding [see Warnings and Precautions (5.2)] • Thrombotic thrombocytopenic purpura [see Warnings ...
-
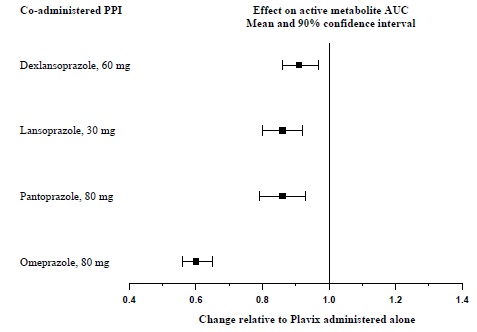
7 DRUG INTERACTIONS7.1 CYP2C19 Inducers - Since clopidogrel is metabolized to its active metabolite partly by CYP2C19, use of drugs that induce the activity of this enzyme would be expected to result in increased ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data from cases reported in published literature and postmarketing surveillance with clopidogrel use in pregnant women have not identified any ...
-
10 OVERDOSAGEPlatelet inhibition by clopidogrel tablets is irreversible and will last for the life of the platelet. Overdose following clopidogrel administration may result in bleeding complications. A single ...
-
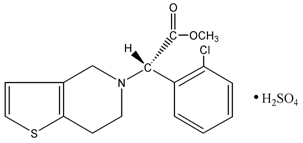
11 DESCRIPTIONClopidogrel is a thienopyridine class inhibitor of P2Y12 ADP platelet receptors. Chemically it is methyl (+)-(S)-α-(2- chlorophenyl)-6,7-dihydrothieno[3,2-c]pyridine-5(4H)-acetate sulfate (1:1) ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Clopidogrel is an inhibitor of platelet activation and aggregation through the irreversible binding of its active metabolite to the P2Y12 class of ADP receptors on ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - There was no evidence of tumorigenicity when clopidogrel was administered for 78 weeks to mice and 104 weeks to rats at dosages up to ...
-
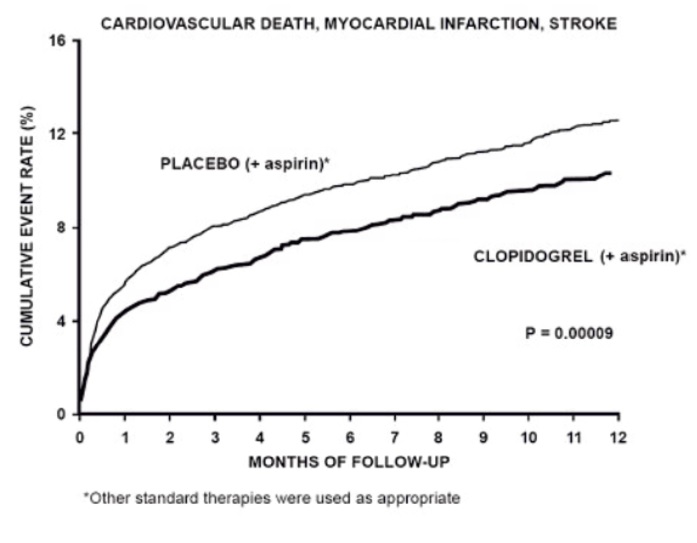
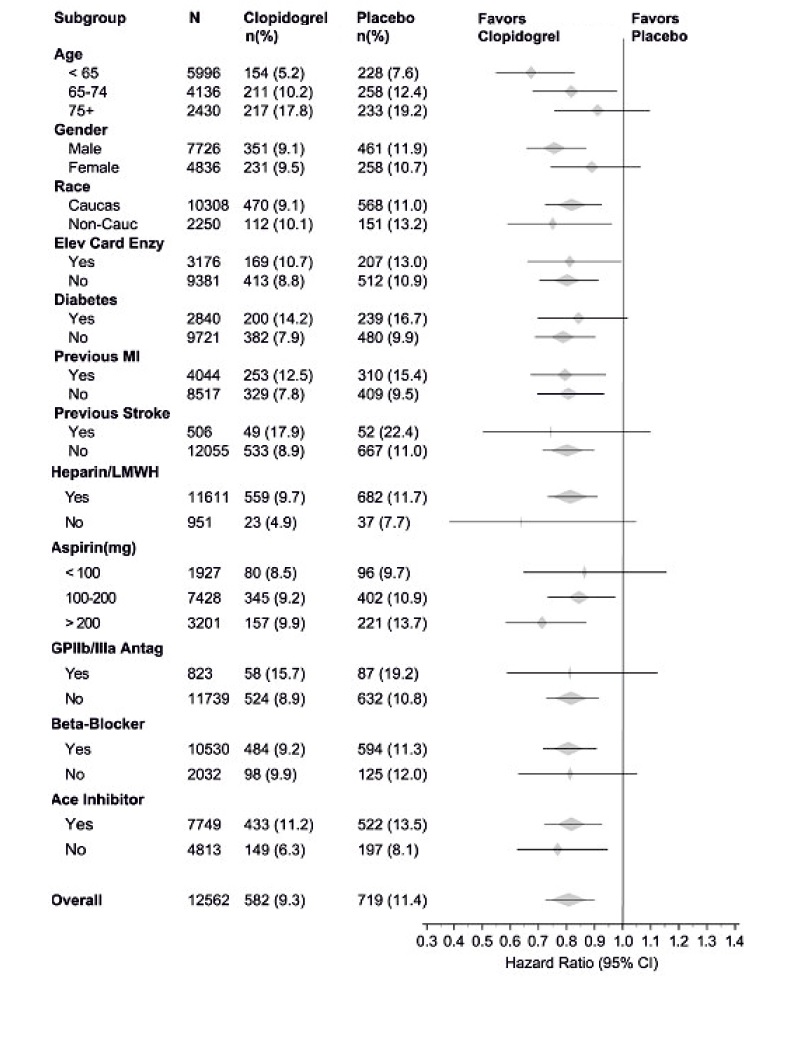
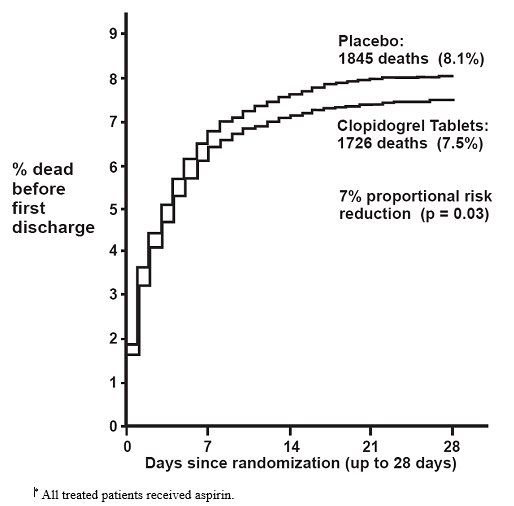
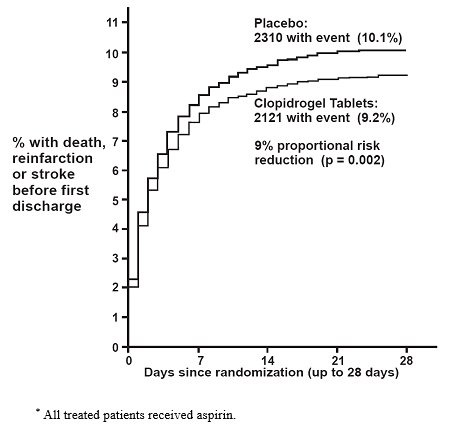
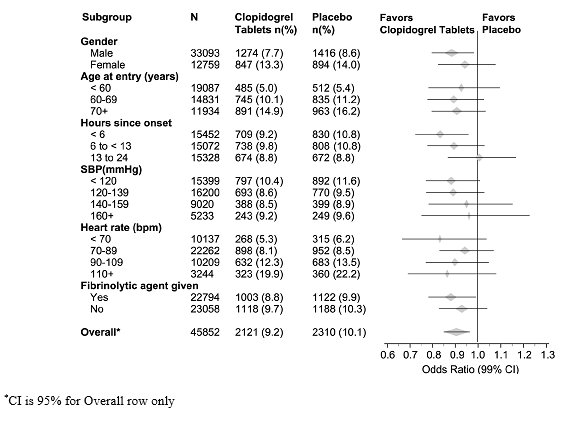
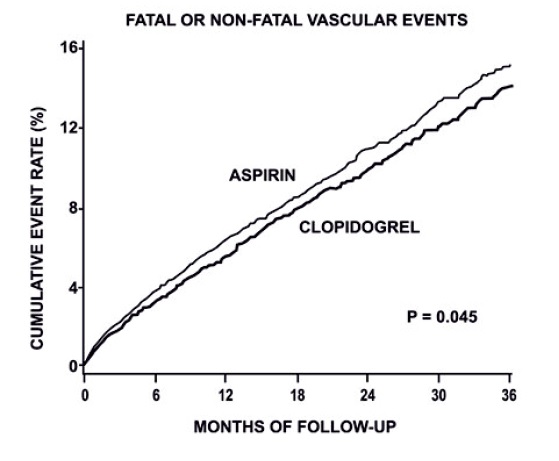
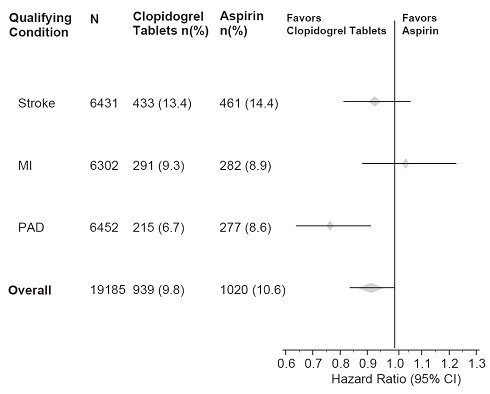
14 CLINICAL STUDIES14.1 Acute Coronary Syndrome - CURE - The CURE study included 12,562 patients with ACS without ST-elevation(UA or NSTEMI)and presenting within 24 hours of onset of the most recent episode of ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGProduct: 50090-7467 - NDC: 50090-7467-0 90 TABLET in a BOTTLE
-
17 PATIENT COUNSELING INFORMATIONAdvise patients to read FDA approved patient labeling (Medication Guide). Discontinuation - Advise patients not to discontinue clopidogrel tablets without first discussing it with the ...
-
Medication GuideClopidogrel (kloe pid′ oh grel) tablets - Read this Medication Guide before you start taking clopidogrel tablets and each time you get a refill. There may be new information. This Medication ...
-
CLOPIDOGREL BISULFATE

-
INGREDIENTS AND APPEARANCEProduct Information