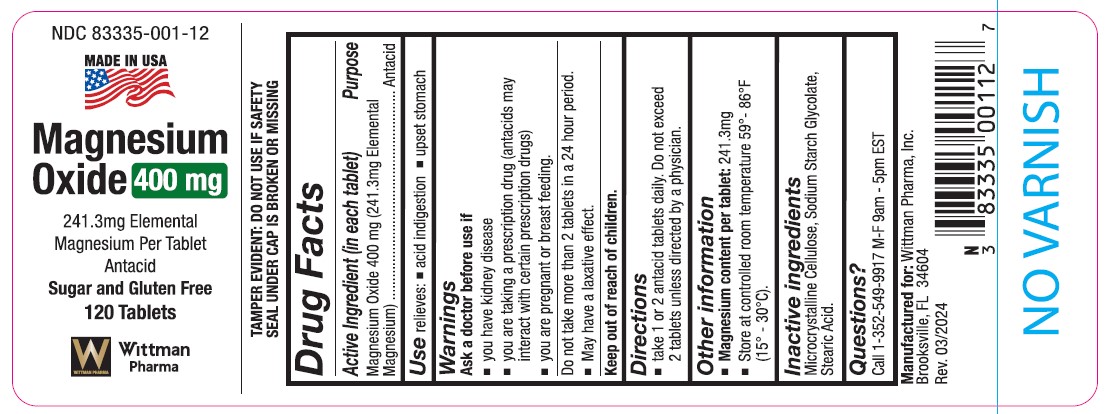
Label: MAGNESIUM OXIDE tablet
- NDC Code(s): 83335-001-12
- Packager: Wittman Pharma, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 4, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DOSAGE & ADMINISTRATIONTablet taken Orally - Do not take more than 2 tablets in a 24 hour period
-
WARNINGSAsk a doctor before use if: * you have kidney disease - * you are taking a prescription drug (antacids may interact with certain prescription drugs) * you are pregnant or breast feeding
-
INACTIVE INGREDIENTMicrocrystaline Cellulose, Sodium Starch Glycolate, Stearic Acid
-
INDICATIONS & USAGErelieves acid indigestion and upset stomach
-
KEEP OUT OF REACH OF CHILDRENKeep out of reach of children
-
PURPOSEAntacid
-
ACTIVE INGREDIENTActive Ingredient (in each tablet) Magnesium Oxide 400 mg (241.3 mg Elemental Magnesium)
-
Drug FactsACTIVE INGREDIENT (In each tablet) Magnesium Oxide 400mg (241.3mg elemental magnesium) Purpose - Antacid - USES - relieves - acid indigestion - upset stomach - WARNINGS - Ask a doctor before use ...
-
INGREDIENTS AND APPEARANCEProduct Information