Label: SARASOTA MEMORIAL AMENITY- alcohol and sodium monofluorophosphate kit
- NDC Code(s): 42555-060-45, 59448-004-01, 59448-600-00
- Packager: ASP Global, LLc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 15, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
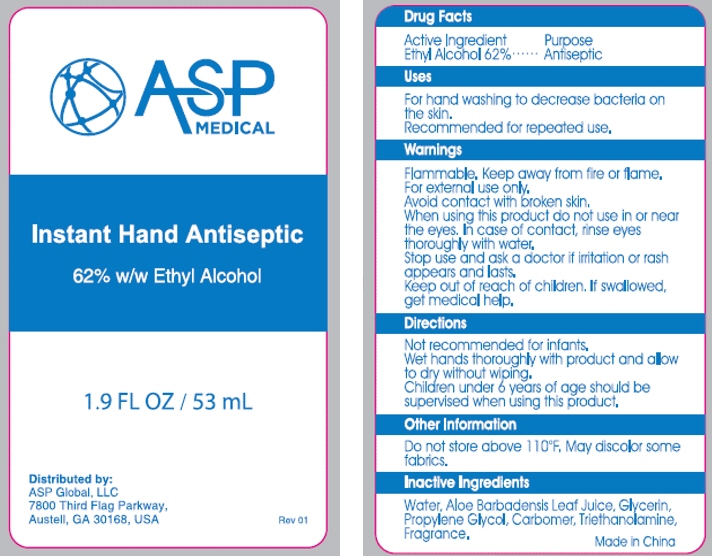
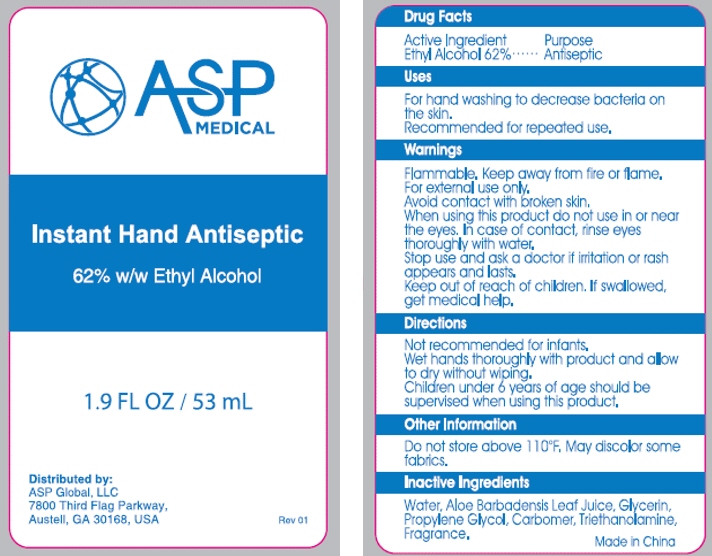
- Active ingredient
- Purpose
- Use
- Warnings
-
Directions
adults and children 2 years of age and older brush teeth thoroughly, preferably after each meal or at least twice a day, or as directed by a dentist or physician children 2 to 6 years use only a pea sized amount and supervise child's brushing and rinsing (to minimize swallowing) children under 2 years ask a dentist or physician - Inactive ingredients
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
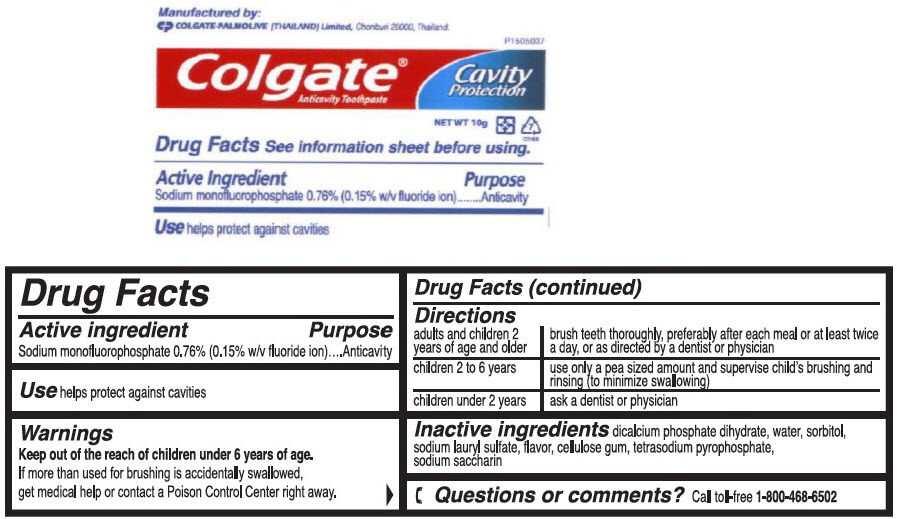
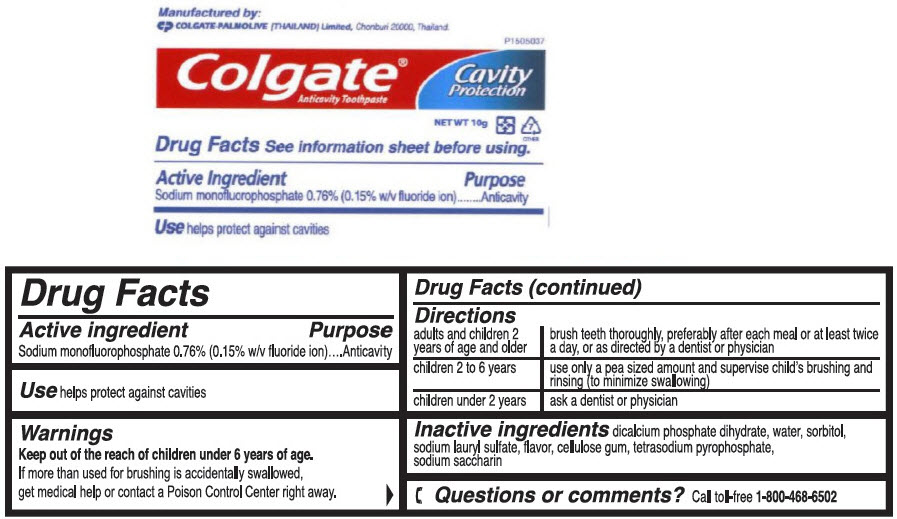
- Active Ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other Information
- Inactive Ingredients
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 53 mL Bottle Label
- PRINCIPAL DISPLAY PANEL - 10 g Tube Label
-
PRINCIPAL DISPLAY PANEL - Kit Label
SARASOTA
MEMORIAL
HEALTH CARE SYSTEMContents/Origins:
1.9 fl. oz. Hand Sanitizer
Toothbrush, Sleep Mask, Ear Plugs, Facial Tissue, Patient
Hand Hygiene Education Card, Paper Bag and Kit Case:
Made in ChinaToothpaste: Made in Thailand
Lot #: XMMDDFC
Exp: YYYY-MM-DDDistributed by:
ASP Global, LLC
7800 Third Flag Parkway, Austell, GA 30168, USAREV 02
SARASOTA
MEMORIAL
HEALTH CARE SYSTEMItem #: SARASOTA01
Description: AMENITY KIT, SARASOTA
MEM WELCOME
PO #:
Lot #: XMMDDFC
Exp: YYYY-MM-DD
Qty: 30 KIT/CS
Carton #: XXX of XXX
Net Wt.: XX KG
Gross Wt.: XX KG
Cubic Dimensions: XX x XX x XX CM
Assembled in ChinaComponents Made in China:
- Hand Sanitizer
- Toothbrush
- Sleep Mask
- Ear Plugs
- Facial Tissue
- Paper Bag
- Kit Case
Component Made in Thailand:
- Toothpaste
SARASOTA
MEMORIAL
HEALTH CARE SYSTEM
-
INGREDIENTS AND APPEARANCE
SARASOTA MEMORIAL AMENITY
alcohol and sodium monofluorophosphate kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59448-600 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59448-600-00 30 in 1 BOX 10/08/2019 1 1 in 1 BAG Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE, PLASTIC 53 mL Part 2 1 TUBE 10 g Part 1 of 2 INSTANT HAND ANTISEPTIC
alcohol gelProduct Information Item Code (Source) NDC:59448-004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 62 mL in 100 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Aloe Vera Leaf (UNII: ZY81Z83H0X) Glycerin (UNII: PDC6A3C0OX) Propylene Glycol (UNII: 6DC9Q167V3) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) Trolamine (UNII: 9O3K93S3TK) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59448-004-01 53 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part333E 11/27/2018 Part 2 of 2 COLGATE ANTICAVITY
sodium monofluorophosphate paste, dentifriceProduct Information Item Code (Source) NDC:42555-060 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM MONOFLUOROPHOSPHATE (UNII: C810JCZ56Q) (Fluoride Ion - UNII:Q80VPU408O) Fluoride Ion 7.6 mg in 1 g Inactive Ingredients Ingredient Name Strength DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) Water (UNII: 059QF0KO0R) Sorbitol (UNII: 506T60A25R) Sodium Lauryl Sulfate (UNII: 368GB5141J) SODIUM PYROPHOSPHATE (UNII: O352864B8Z) SACCHARIN SODIUM (UNII: SB8ZUX40TY) Product Characteristics Color WHITE Score Shape Size Flavor MINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42555-060-45 10 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part355 06/04/2009 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 10/08/2019 Labeler - ASP Global, LLc (080361159) Establishment Name Address ID/FEI Business Operations Shengzhou Kingbird Travel Products Co., Ltd. 560219293 PACK(59448-600) , LABEL(59448-600) Establishment Name Address ID/FEI Business Operations Colgate-Palmolive (Thailand) LTD 672044552 MANUFACTURE(59448-600) Establishment Name Address ID/FEI Business Operations Nantong Health & Beyond Hygienic Products Inc. 421280161 MANUFACTURE(59448-600)