Label: NEOMYCIN SULFATE tablet
- NDC Code(s): 73473-901-05
- Packager: Solaris Pharma Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
BOXED WARNING
(What is this?)
BOXED WARNING
WARNINGS
SYSTEMIC ABSORPTION OF NEOMYCIN OCCURS FOLLOWING ORAL ADMINISTRATION AND TOXIC REACTIONS MAY OCCUR.
Patients treated with neomycin should be under close clinical observation because of the potential toxicity associated with their use. NEUROTOXICITY (INCLUDING OTOTOXICITY) AND NEPHROTOXICITY FOLLOWING THE ORAL USE OF NEOMYCIN SULFATE HAVE BEEN REPORTED, EVEN WHEN USED IN RECOMMENDED DOSES. THE POTENTIAL FOR NEPHROTOXICITY, PERMANENT BILATERAL AUDITORY OTOTOXICITY AND SOMETIMES VESTIBULAR TOXICITY IS PRESENT IN PATIENTS WITH NORMAL RENAL FUNCTION WHEN TREATED WITH HIGHER DOSES OF NEOMYCIN AND/OR FOR LONGER PERIODS THAN RECOMMENDED. Serial, vestibular and audiometric tests, as well as tests of renal function, should be performed (especially in high-risk patients). THE RISK OF NEPHROTOXICITY AND OTOTOXICITY IS GREATER IN PATIENTS WITH IMPAIRED RENAL FUNCTION. Ototoxicity is often delayed in onset and patients developing cochlear damage will not have symptoms during therapy to warn them of developing eighth nerve destruction and total or partial deafness may occur long after neomycin has been discontinued.
Neuromuscular blockage and respiratory paralysis have been reported following the oral use of neomycin. The possibility of the occurrence of neuromuscular blockage and respiratory paralysis should be considered if neomycin is administered, especially to patients receiving anesthetics, neuromuscular blocking agents such as tubocurarine, succinylcholine, decamethonium, or in patients receiving massive transfusions of citrate anticoagulated blood. If blockage occurs, calcium salts may reverse these phenomena but mechanical respiratory assistance may be necessary.
Concurrent and/or sequential systemic, oral or topical use of other aminoglycosides, including paromomycin and other potentially nephrotoxic and/or neurotoxic drugs such as bacitracin, cisplatin, vancomycin, amphotericin B, polymyxin B, colistin and viomycin, should be avoided because the toxicity may be additive.
Other factors which increase the risk of toxicity are advanced age and dehydration.
The concurrent use of neomycin with potent diuretics such as ethacrynic acid or furosemide should be avoided, since certain diuretics by themselves may cause ototoxicity. In addition, when administered intravenously, diuretics may enhance neomycin toxicity by altering the antibiotic concentration in serum and tissue.
-
DESCRIPTION
Neomycin Sulfate Tablets, USP, for oral administration, contain neomycin which is an antibiotic obtained from the metabolic products of the actinomycete Streptomyces fradiae.
Structurally, neomycin sulfate may be represented as follows:
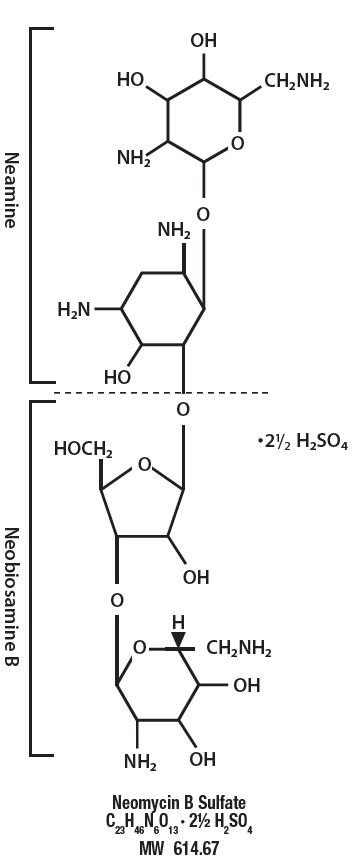
Chemically, it is 0-2, 6-diamino-2, 6-dideoxy-α D-glucopyranosyl-(1→3)- 0-β-D-ribofuranosyl- (1→5)- 0-[2,6-diamino-2, 6-dideoxy-α-D-glucopyranosyl -(1→4)]-2-deoxy-D-streptamine. Neomycin B is identical except that the α-D-glucopyranosyl residue in the neobiosamine moiety is β-L-idopyranosyl.
Inactive Ingredients: Calcium Stearate, Povidone.
-
CLINICAL PHARMACOLOGY
Neomycin sulfate is poorly absorbed from the normal gastrointestinal tract.The small absorbed fraction is rapidly distributed in the tissues and is excreted by the kidney in keeping with the degree of kidney function. The unabsorbed portion of the drug (approximately 97%) is eliminated unchanged in the feces.
Growth of most intestinal bacteria is rapidly suppressed following oral administration of neomycin sulfate, with the suppression persisting for 48-72 hours. Nonpathogenic yeasts and occasionally resistant strains of Enterobacter aerogenes (formerly Aerobacter aerogenes) replace the intestinal bacteria.
As with other aminoglycosides, the amount of systemically absorbed neomycin transferred to the tissues increases cumulatively with each repeated dose administered until a steady state is achieved. The kidney functions as the primary excretory path as well as the tissue binding site, with the highest concentration found in the renal cortex. With repeated dosings, progressive accumulation also occurs in the inner ear. Release of tissue-bound neomycin occurs slowly over a period of several weeks after dosing has been discontinued.
Protein binding studies have shown that the degree of aminoglycoside protein binding is low and, depending upon the methods used for testing, this may be between 0% and 30%.
Microbiology
In vitro tests have demonstrated that neomycin is bactericidal and acts by inhibiting the synthesis of protein in susceptible bacterial cells. It is effective primarily against gram-negative bacilli but does have some activity against gram-positive organisms. Neomycin is active in vitro against Escherichia coli and the Klebsiella-Enterobacter group. Neomycin is not active against anaerobic bowel flora.
Susceptibility Testing
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https:// www.fda.gov/STIC. -
INDICATIONS AND USAGE
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Neomycin Sulfate Tablets, USP and other antibacterial drugs, Neomycin Sulfate Tablets, USP should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Suppression of Intestinal Bacteria
Neomycin Sulfate Tablets, USP are indicated as adjunctive therapy as part of a regimen for the suppression of the normal bacterial flora of the bowel, e.g. preoperative preparation of the bowel. It is given concomitantly with erythromycin enteric-coated base (seeDOSAGE AND ADMINISTRATION section).
-
CONTRAINDICATIONS
Neomycin sulfate oral preparations are contraindicated in the presence of intestinal obstruction and in individuals with a history of hypersensitivity to the drug.
Patients with a history of hypersensitivity or serious toxic reaction to other aminoglycosides may have a cross-sensitivity to neomycin.
Neomycin sulfate oral preparations are contraindicated in patients with inflammatory or ulcerative gastrointestinal disease because of the potential for enhanced gastrointestinal absorption of neomycin.
-
WARNINGS
(see boxed WARNINGS)
Additional manifestations of neurotoxicity may include numbness, skin tingling, muscle twitching and convulsions.
The risk of hearing loss continues after drug withdrawal.
Aminoglycosides can cause fetal harm when administered to a pregnant woman. Aminoglycoside antibiotics cross the placenta and there have been several reports of total irreversible bilateral congenital deafness in children whose mothers received streptomycin during pregnancy. Although serious side effects to fetus or newborn have not been reported in the treatment of pregnant women with other aminoglycosides, the potential for harm exists. Animal reproduction studies of neomycin have not been conducted. If neomycin is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus.
Risk of Ototoxicity Due to Mitochondrial DNA Variants
Cases of ototoxicity with aminoglycosides have been observed in patients with certain variants in the mitochondrially encoded 12S rRNA gene ( MT-RNR1), particularly the m.1555A>G variant. Ototoxicity occurred in some patients even when their aminoglycoside serum levels were within the recommended range. Mitochondrial DNA variants are present in less than 1% of the general US population, and the proportion of the variant carriers who may develop ototoxicity as well as the severity of ototoxicity is unknown. In case of known maternal history of ototoxicity due to aminoglycoside use or a known mitochondrial DNA variant in the patient, consider alternative treatments other than aminoglycosides unless the increased risk of permanent hearing loss is outweighed by the severity of infection and lack of safe and effective alternative therapies.
-
PRECAUTIONS
General
Prescribing Neomycin Sulfate Tablets, USP in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
As with other antibiotics, use of oral neomycin may result in overgrowth of non-susceptible organisms, particularly fungi. If this occurs, appropriate therapy should be instituted.
Neomycin is quickly and almost totally absorbed from body surfaces (except the urinary bladder) after local irrigation and when applied topically in association with surgical procedures. Delayed-onset irreversible deafness, renal failure and death due to neuromuscular blockade (regardless of the status of renal function) have been reported following irrigation of both small and large surgical fields with minute quantities of neomycin.
Cross-allergenicity among aminoglycosides has been demonstrated.
Aminoglycosides should be used with caution in patients with muscular disorders such as myasthenia gravis or parkinsonism since these drugs may aggravate muscle weakness because of their potential curare-like effect on the neuromuscular junction.
Small amounts of orally administered neomycin are absorbed through intact intestinal mucosa.
There have been many reports in the literature of nephrotoxicity and/or ototoxicity with the oral use of neomycin. If renal insufficiency develops during oral therapy, consideration should be given to reducing the drug dosage or discontinuing therapy.
An oral neomycin dose of 12 grams per day produces malabsorption syndrome for a variety of substances, including fat, nitrogen, cholesterol, carotene, glucose, xylose, lactose, sodium, calcium, cyanocobalamin and iron.
Orally administered neomycin increases fecal bile acid excretion and reduces intestinal lactase activity.
Information for Patients
Patients should be counseled that antibacterial drugs, including Neomycin Sulfate Tablets, USP, should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When Neomycin Sulfate Tablets, USP are prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by neomycin sulfate tablets or other antibacterial drugs in the future.
Before administering the drug, patients or members of their families should be informed of possible toxic effects on the eighth nerve. The possibility of acute toxicity increases in premature infants and neonates.
Laboratory Tests
Patients with renal insufficiency may develop toxic neomycin blood levels unless doses are properly regulated. If renal insufficiency develops during treatment, the dosage should be reduced or the antibiotic discontinued. To avoid nephrotoxicity and eighth nerve damage associated with high doses and prolonged treatment, the following should be performed prior to and periodically during therapy: urinalysis for increased excretion of protein, decreased specific gravity, casts and cells; renal function tests such as serum creatinine, BUN or creatinine clearance; tests of the vestibulocochlearis nerve (eighth cranial nerve) function.
Serial, vestibular and audiometric tests should be performed (especially in high-risk patients). Since elderly patients may have reduced renal function which may not be evident in the results of routine screening tests such as BUN or serum creatinine, a creatinine clearance determination may be more useful.
Drug Interactions
Caution should be taken in concurrent or serial use of other neurotoxic and/or nephrotoxic drugs because of possible enhancement of the nephrotoxicity and/or ototoxicity of neomycin (see boxed WARNINGS) .
Caution should also be taken in concurrent or serial use of other aminoglycosides and polymyxins because they may enhance neomycin’s nephrotoxicity and/or ototoxicity and potentiate neomycin sulfate’s neuromuscular blocking effects.
Oral neomycin inhibits the gastrointestinal absorption of penicillin V, oral vitamin B-12, methotrexate and 5-fluorouracil. The gastrointestinal absorption of digoxin also appears to be inhibited. Therefore, digoxin serum levels should be monitored.
Oral neomycin sulfate may enhance the effect of coumarin in anticoagulants by decreasing vitamin K availability.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term animal studies have been performed with neomycin sulfate to evaluate carcinogenic or mutagenic potential or impairment of fertility.
Nursing Mothers
It is not known whether neomycin is excreted in human milk, but it has been shown to be excreted in cow milk following a single intramuscular injection. Other aminoglycosides have been shown to be excreted in human milk. Because of the potential for serious adverse reactions from the aminoglycosides in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
The safety and efficacy of oral neomycin sulfate in patients less than 18 years of age have not been established. If treatment of a patient less than 18 years of age is necessary, neomycin should be used with caution and the period of treatment should not exceed two weeks because of absorption from the gastrointestinal tract.
-
ADVERSE REACTIONS
The most common adverse reactions to oral neomycin sulfate are nausea, vomiting and diarrhea. The “Malabsorption Syndrome” characterized by increased fecal fat, decreased serum carotene and fall in xylose absorption has been reported with prolonged therapy. Nephrotoxicity, ototoxicity and neuromuscular blockage have been reported (see boxed WARNINGS and PRECAUTIONS sections).
To report SUSPECTED ADVERSE REACTIONS, contact Solaris Pharma Corporation at 1-833-919-0527 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
-
OVERDOSAGE
Because of low absorption, it is unlikely that acute overdosage would occur with oral neomycin sulfate. However, prolonged administration could result in sufficient systemic drug levels to produce neurotoxicity, ototoxicity and/or nephrotoxicity.
Hemodialysis will remove neomycin sulfate from the blood.
-
DOSAGE AND ADMINISTRATION
To minimize the risk of toxicity, use the lowest possible dose and the shortest possible treatment period to control the condition. Treatment for periods longer than two weeks is not recommended.
Hepatic Coma
For use as an adjunct in the management of hepatic coma, the recommended dose is 4 to 12 grams per day given in the following regimen:
- Withdraw protein from diet. Avoid use of diuretic agents.
- Give supportive therapy, including blood products, as indicated.
- Give Neomycin Sulfate Tablets, USP in doses of 4 to 12 grams of neomycin sulfate per day (eight to 24 tablets) in divided doses. Treatment should be continued over a period of five to six days, during which time protein should be returned incrementally to the diet.
- If less potentially toxic drugs cannot be used for chronic hepatic insufficiency, neomycin in doses of up to four grams daily (eight tablets per day) may be necessary. The risk for the development of neomycin-induced toxicity progressively increases when treatment must be extended to preserve the life of a patient with hepatic encephalopathy who has failed to fully respond. Frequent periodic monitoring of these patients to ascertain the presence of drug toxicity is mandatory (see PRECAUTIONS) . Also, neomycin serum concentrations should be monitored to avoid potentially toxic levels. The benefits to the patient should be weighed against the risks of nephrotoxicity, permanent ototoxicity and neuromuscular blockade following the accumulation of neomycin in the tissues.
Preoperative Prophylaxis for Elective Colorectal Surgery
Listed below is an example of a recommended bowel preparation regimen. A proposed surgery time of 8:00 a.m. has been used.
Pre-op Day 3: Minimum residue or clear liquid diet. Bisacodyl, 1 tablet orally at 6:00 p.m.
Pre-op Day 2: Minimum residue or clear liquid diet. Magnesium sulfate, 30 mL, 50% solution (15 g) orally at 10:00 a.m., 2:00 p.m., and 6:00 p.m. Enema at 7:00 p.m. and 8:00 p.m.
Pre-op Day 1: Clear liquid diet. Supplemental (IV) fluids as needed. Magnesium sulfate, 30 mL, 50% solution (15 g) orally at 10:00 a.m. and 2:00 p.m. Neomycin sulfate (1 g) and erythromycin base (1 g) orally at 1:00 p.m., 2:00 p.m. and 11:00 p.m. No enema.
Day of Operation: Patient evacuates rectum at 6:30 a.m. for scheduled operation at 8:00 a.m.
-
HOW SUPPLIED
Neomycin Sulfate Tablets, USP, 500 mg (equivalent to 350 mg of neomycin base per tablet) are available as round, off-white, unscored tablets, debossed “500” and “PT”.
Supplied as: NDC 73473-901-05 for 100 tablets per bottle.
Store at 20º to 25ºC (68º to 77ºF) [see USP Controlled Room Temperature].
Dispense in tight containers as defined in the USP/NF.
Manufactured in Canada.
Distributed by:
Solaris Pharma Corporation
Bridgewater, NJ 08807Revised: November 2023
NST-SL-PI-01
2000016178 - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NEOMYCIN SULFATE
neomycin sulfate tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:73473-901 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NEOMYCIN SULFATE (UNII: 057Y626693) (NEOMYCIN - UNII:I16QD7X297) NEOMYCIN SULFATE 500 mg Inactive Ingredients Ingredient Name Strength CALCIUM STEARATE (UNII: 776XM7047L) POVIDONE (UNII: FZ989GH94E) Product Characteristics Color white (OFF-WHITE) Score no score Shape ROUND Size 11mm Flavor Imprint Code 500;pt Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73473-901-05 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/01/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA065220 06/01/2023 Labeler - Solaris Pharma Corporation (079904672) Registrant - XGen Pharmaceuticals DJB, Inc. (117380305)


