Label: IBUPROFEN tablet, film coated
-
Contains inactivated NDC Code(s)
NDC Code(s): 58624-7007-0, 58624-7009-0, 58624-7011-0 - Packager: Shandong Xinhua Pharmaceutical Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 3, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
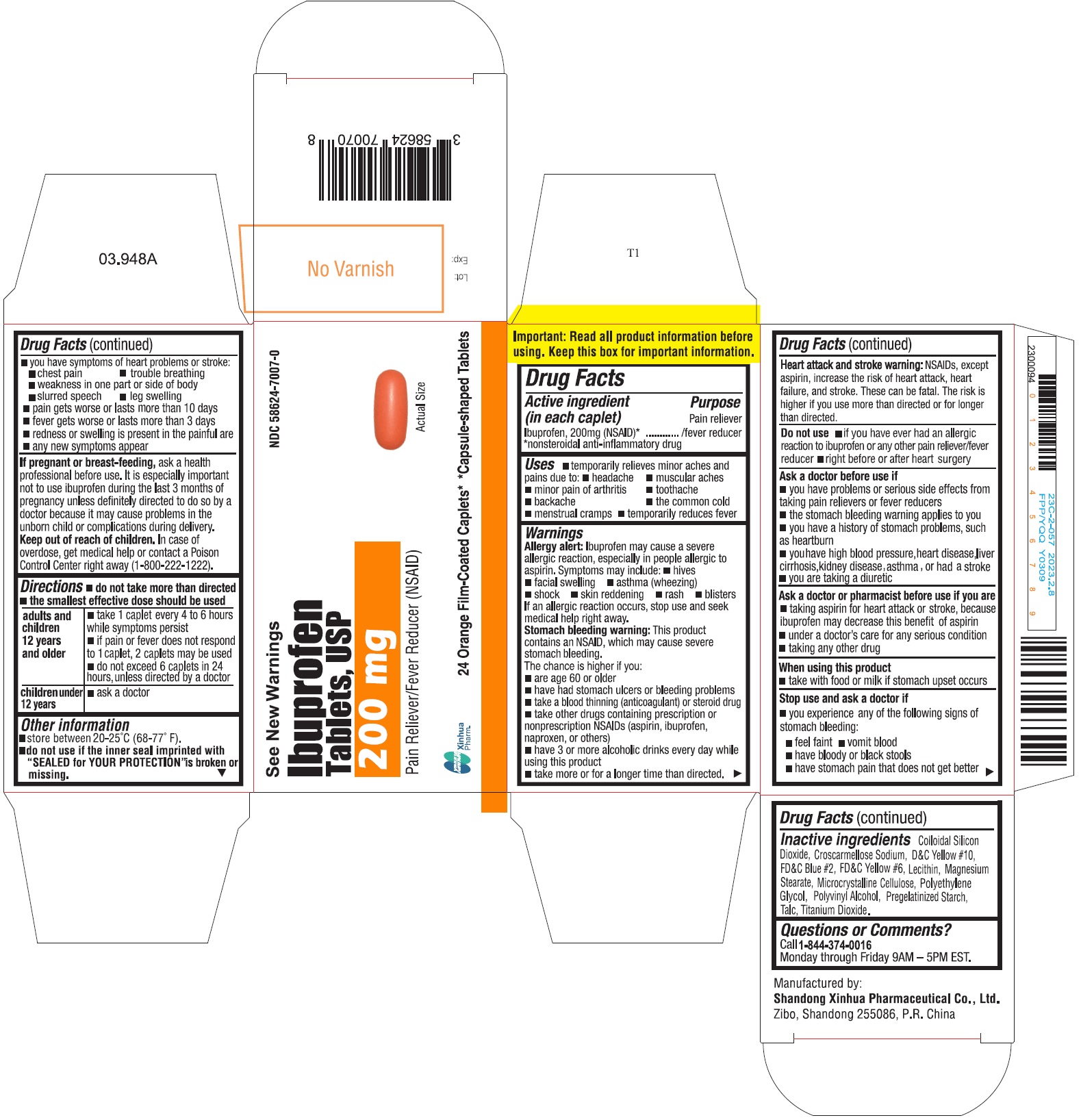
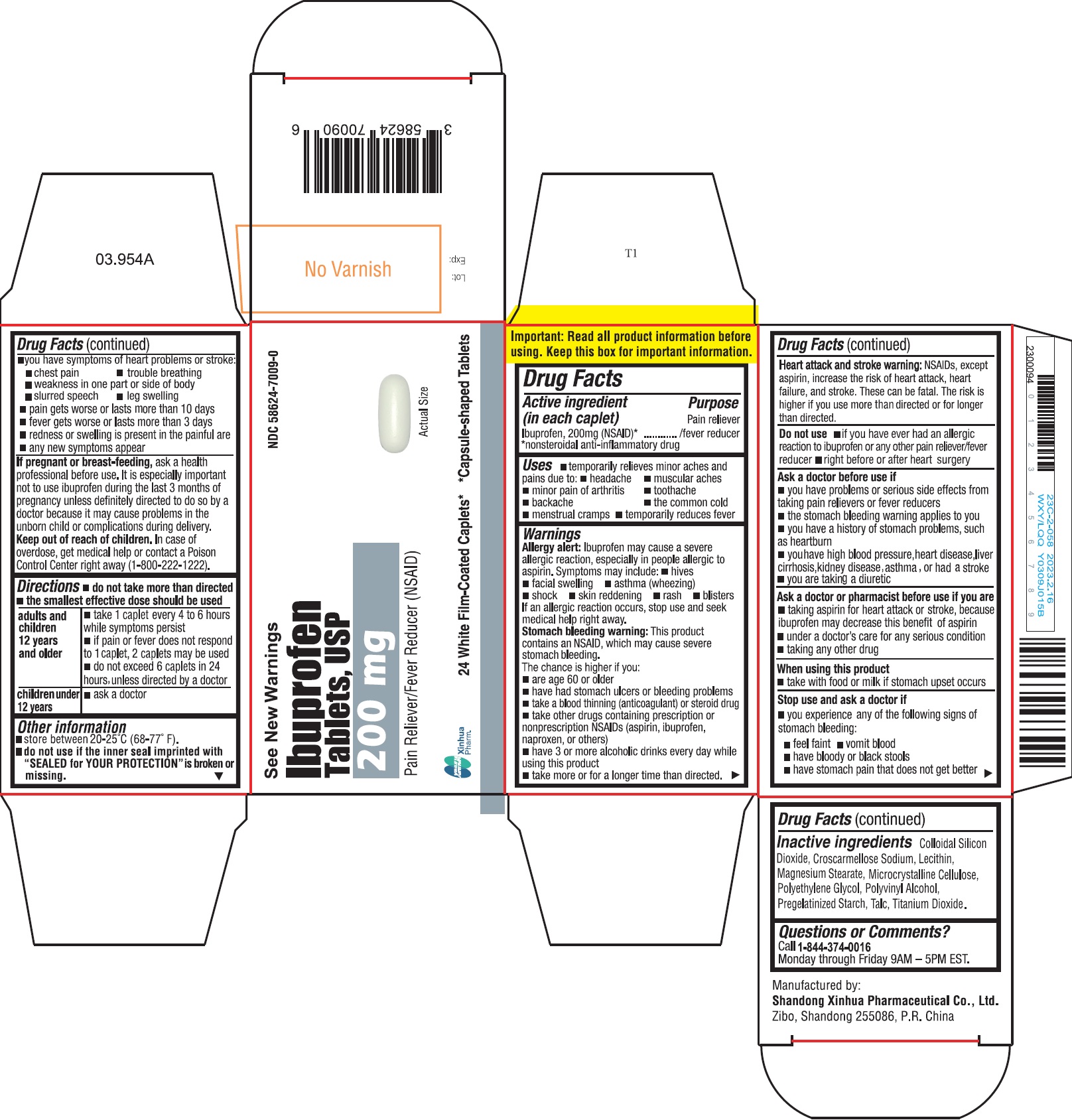
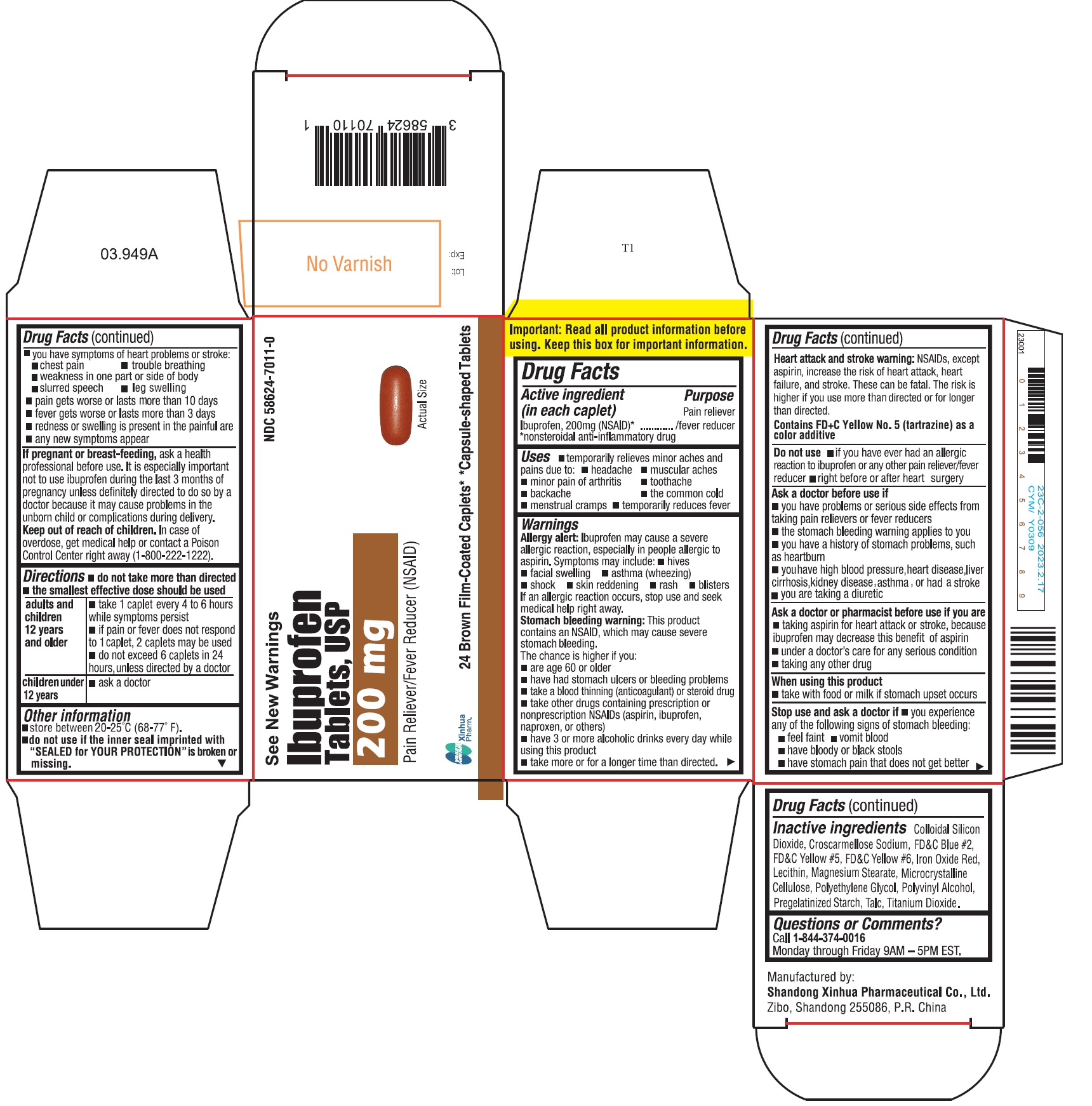
ImportantRead all product information before using. Keep this box for important information.
-
SPL UNCLASSIFIED SECTIONDrug Facts
-
Active ingredient (in each caplet)Ibuprofen 200 mg (NSAID)* *nonsteroidal anti-inflammatory drug
-
PurposePain reliever/fever reducer
-
Usestemporarily relieves minor aches and pains due to: headache - muscular aches - minor pain of arthritis - toothache - backache - the common cold - menstrual ...
-
WarningsAllergy alert - Ibuprofen may cause a severe allergic reaction, especially in people allergic to aspirin. Symptoms may include: hives - facial swelling - asthma (wheezing) shock - skin ...
-
Directionsdo not take more than directed - the smallest effective dose should be used - adults and children 12 years and older - take 1 caplet every 4 to 6 hours while symptoms persist - if pain or fever ...
-
Other informationstore between 20-25°C (68-77°F) do not use if the inner seal imprinted with "SEALED for YOUR PROTECTION" is broken or missing
- INACTIVE INGREDIENT
-
Questions or comments?Call - 1-844-374-0016 Monday through Friday 9AM - 5PM EST.
-
PRINCIPAL DISPLAY PANELIbuprofen tablets are available in the following colors and sizes: Orange, Capsule-shaped Tablet, debossed with BI 03 - Bottles of 24 NDC 58624-7007-0 - White, Capsule-shaped Tablet, debossed ...
-
INGREDIENTS AND APPEARANCEProduct Information