Label: ISONIAZID tablet
- NDC Code(s): 64950-216-10, 64950-217-03, 64950-217-10
- Packager: Genus Lifesciences
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
WARNING
Severe and sometimes fatal hepatitis associated with isoniazid therapy has been reported and may occur or may develop even after many months of treatment. The risk of developing hepatitis is age related. Approximate case rates by age are: less than 1 per 1,000 for persons under 20 years of age, 3 per 1,000 for persons in the 20 to 34 year age group, 12 per 1,000 for persons in the 35 to 49 year age group, 23 per 1,000 for persons in the 50 to 64 year age group and 8 per 1,000 for persons over 65 years of age. The risk of hepatitis is increased with daily consumption of alcohol. Precise data to provide a fatality rate for isoniazid-related hepatitis is not available; however, in a U.S. Public Health Service Surveillance Study of 13,838 persons taking isoniazid, there were 8 deaths among 174 cases of hepatitis.
Therefore, patients given isoniazid should be carefully monitored and interviewed at monthly intervals. For persons 35 and older, in addition to monthly symptom reviews, hepatic enzymes (specifically, AST and ALT [formerly SGOT and SGPT, respectively]) should be measured prior to starting isoniazid therapy and periodically throughout treatment. Isoniazid-associated hepatitis usually occurs during the first three months of treatment. Usually, enzyme levels return to normal despite continuance of drug, but in some cases progressive liver dysfunction occurs. Other factors associated with an increased risk of hepatitis include daily use of alcohol, chronic liver disease and injection drug use. A recent report suggests an increased risk of fatal hepatitis associated with isoniazid among women, particularly black and Hispanic women. The risk may also be increased during the post partum period. More careful monitoring should be considered in these groups, possibly including more frequent laboratory monitoring. If abnormalities of liver function exceed three to five times the upper limit of normal, discontinuation of isoniazid should be strongly considered. Liver function tests are not a substitute for a clinical evaluation at monthly intervals or for the prompt assessment of signs or symptoms of adverse reactions occurring between regularly scheduled evaluations. Patients should be instructed to immediately report signs or symptoms consistent with liver damage or other adverse effects. These include any of the following: unexplained anorexia, nausea, vomiting, dark urine, icterus, rash, persistent paresthesias of the hands and feet, persistent fatigue, weakness or fever of greater than 3 days duration and/or abdominal tenderness, especially right upper quadrant discomfort. If these symptoms appear or if signs suggestive of hepatic damage are detected, isoniazid should be discontinued promptly, since continued use of the drug in these cases has been reported to cause a more severe form of liver damage.
Patients with tuberculosis who have hepatitis attributed to isoniazid should be given appropriate treatment with alternative drugs. If isoniazid must be reinstituted, it should be reinstituted only after symptoms and laboratory abnormalities have cleared. The drug should be restarted in very small and gradually increasing doses and should be withdrawn immediately if there is any indication of recurrent liver involvement.
Preventive treatment should be deferred in persons with acute hepatic diseases.
Close -
DESCRIPTIONIsoniazid, USP is an antibacterial available as 100 mg and 300 mg tablets for oral administration. Each tablet also contains as inactive ingredients: colloidal silicon dioxide, crospovidone ...
-
CLINICAL PHARMACOLOGYWithin 1 to 2 hours after oral administration, isoniazid produces peak blood levels which decline to 50 percent or less within 6 hours. It diffuses readily into all body fluids (cerebrospinal ...
-
INDICATIONS AND USAGEIsoniazid tablets, USP are recommended for all forms of tuberculosis in which organisms are susceptible. However, active tuberculosis must be treated with multiple concomitant anti-tuberculosis ...
-
CONTRAINDICATIONSIsoniazid is contraindicated in patients who develop severe hypersensitivity reactions, including drug-induced hepatitis; previous isoniazid-associated hepatic injury; severe adverse reactions to ...
-
WARNINGSSee the boxed warning.
-
PRECAUTIONSGeneral - All drugs should be stopped and an evaluation made at the first sign of a hypersensitivity reaction. If isoniazid therapy must be reinstituted, the drug should be given only after ...
-
ADVERSE REACTIONSThe most frequent reactions are those affecting the nervous system and the liver. Nervous System Reactions - Peripheral neuropathy is the most common toxic effect. It is dose-related, occurs most ...
-
OVERDOSAGESigns and Symptoms - Isoniazid overdosage produces signs and symptoms within 30 minutes to 3 hours after ingestion. Nausea, vomiting, dizziness, slurring of speech, blurring of vision and visual ...
-
DOSAGE AND ADMINISTRATION(See also INDICATIONS AND USAGE) NOTE - For preventive therapy of tuberculous infection and treatment of tuberculosis, it is recommended that physicians be familiar with the following ...
-
HOW SUPPLIEDIsoniazid tablets, USP are available as follows: 100 mg: White to off-white, round, scored, flat-faced, beveled-edge tablet, debossed with 01 on one side and G over L on the other side. Available ...
-
ReferencesMurphy, R., et al: Annuals of Internal Medicine; 1990: November 15; volume 113: 799-800. Burke, R.F., et al: Res Commun Chem Pathol Pharmacol; 1990: July; vol. 69: 115-118. Fleenor, M.F., et al ...
-
SPL UNCLASSIFIED SECTIONManufactured In Canada By: Patheon Inc. Whitby, ON, Canada L1N 5Z5 - Manufactured For: Genus Lifesciences Inc. Allentown, PA 18102 - Rev. 07/2024
-
PRINCIPAL DISPLAY PANEL - 100 mg Tablet Bottle LabelNDC 64950-216-10 - Isoniazid - Tablets, USP - 100 mg - Rx only - 100 Tablets - Genus - Lifesciences Inc.
-
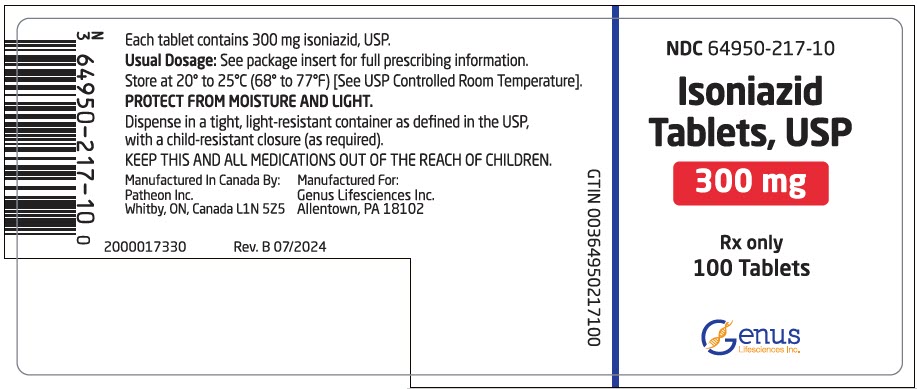
PRINCIPAL DISPLAY PANEL - 300 mg Tablet Bottle LabelNDC 64950-217-10 - Isoniazid - Tablets, USP - 300 mg - Rx only - 100 Tablets - Genus - Lifesciences Inc.
-
INGREDIENTS AND APPEARANCEProduct Information