Label: ENROFLOXACIN AND SILVER SULFADIAZINE OTIC EMULSION- enrofloxacin/silver sulfadiazine emulsion
- NDC Code(s): 11695-7035-2
- Packager: Covetrus
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Animal Drug Application
Drug Label Information
Updated November 22, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONFor Ototopical Use In Dogs - Caution: Federal Law restricts this drug to use by or on the order of a licensed veterinarian. ►Federal law prohibits the extralabel use of this drug in food-producing ...
-
PRODUCT DESCRIPTION:
Each milliliter of Enrofloxacin and Silver Sulfadiazine Otic Emulsion contains: enrofloxacin 5 mg (0.5% w/v), silver sulfadiazine (SSD) 10 mg (1.0% w/v), benzyl alcohol (as a preservative ...
-
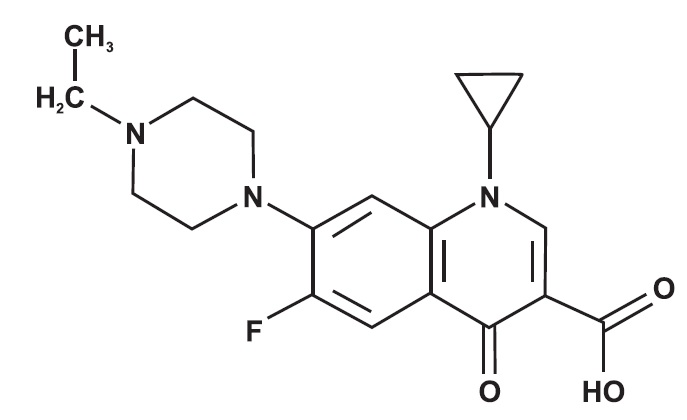
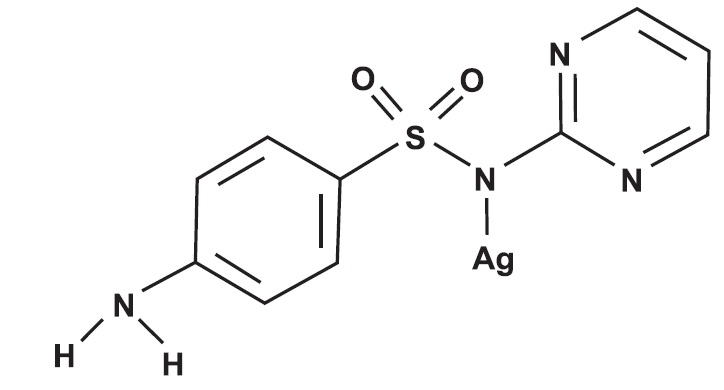
CHEMICAL NOMENCLATURE AND STRUCTURE:
Enrofloxacin - 1-Cyclopropyl-7-(4-ethyl-1-piperazinyl)-6-fluoro-1, 4-dihydro-4-oxo-3-quinolinecarboxylic acid. Silver Sulfadiazine - Benzenesulfonamide ...
-
ACTIONS:
Enrofloxacin, a 4-fluoroquinolone compound, is bactericidal with activity against a broad spectrum of both Gram negative and Gram positive bacteria. Fluoroquinolones elicit their bactericidal ...
-
MICROBIOLOGY:
In clinical field trials, enrofloxacin/silver sulfadiazine otic demonstrated elimination or reduction of clinical signs associated with otitis externa and in vitro activity against cultured ...
-
INDICATIONS:
Enrofloxacin and Silver Sulfadiazine Otic Emulsion is indicated as a treatment for canine otitis externa complicated by bacterial and fungal organisms susceptible to enrofloxacin and/or silver ...
-
EFFECTIVENESS:
Due to its combination of active ingredients, enrofloxacin/silver sulfadiazine otic provides antimicrobial therapy against bacteria and fungi (which includes yeast) commonly encountered in cases ...
-
CONTRAINDICATIONS:
Enrofloxacin and Silver Sulfadiazine Otic Emulsion is contraindicated in dogs with suspected or known hypersensitivity to quinolones and/or sulfonamides.
-
HUMAN WARNINGS:
Not for human use. Keep out of the reach of children. Avoid contact with eyes. In case of contact, immediately flush eyes with copious amounts of water for 15 minutes. In case of dermal contact ...
-
PRECAUTIONS:
The use of Enrofloxacin and Silver Sulfadiazine Otic Emulsion in dogs with perforated tympanic membranes has not been evaluated. Therefore, the integrity of the tympanic membrane should ...
-
ADVERSE REACTIONS:
During clinical trials, 2 of 113 (1.7%) dogs exhibited reactions that may have resulted from treatment with enrofloxacin/silver sulfadiazine otic. Both cases displayed local hypersensitivity ...
-
SAFETY:
General Safety Study: In a target animal safety study, enrofloxacin/silver sulfadiazine otic was administered in both ears of 24 clinically normal beagle dogs at either recommended or ...
-
DOSAGE AND ADMINISTRATION:
Shake well before each use. Tilt head so that the affected ear is presented in an upward orientation. Administer a sufficient quantity of Enrofloxacin and Silver Sulfadiazine Otic Emulsion to coat ...
-
STORAGE:
Store between 4° and 25°C (40 - 77°F). Store in an upright position. Do not store in direct sunlight.
-
HOW SUPPLIED:
Enrofloxacin and Silver Sulfadiazine Otic Emulsion (enrofloxacin/silver sulfadiazine) Size Presentation - 15 mL White LDPE bottle with HDPE cap and dropper
-
REFERENCES:
1. Hooper DC and Wolfson JS. Mechanisms of quinolone action and bacterial killing in quinolone antimicrobial agents. Washington DC, American Society for Microbiology, 2nd ed., 1993: 53-75. 2 ...
-
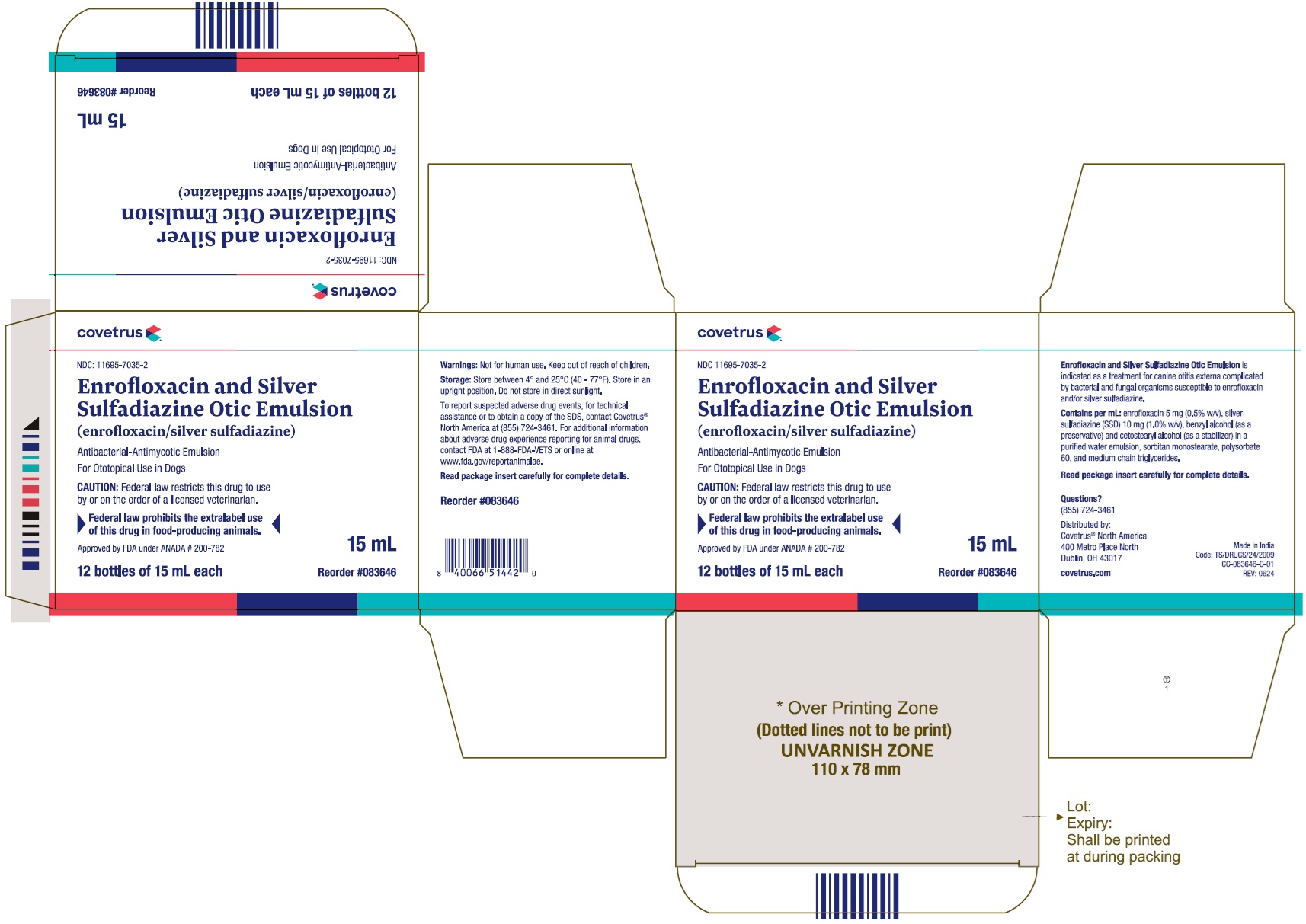
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 11695-7035-2 - Enrofloxacin and Silver Sulfadiazine Otic Emulsion - (enrofloxacin/silver sulfadiazine) Antibacterial-Antimycotic Emulsion - For Ototopical Use in dogs - CAUTION: Federal law ...
-
INGREDIENTS AND APPEARANCEProduct Information