Label: DOXEPIN HYDROCHLORIDE capsule
-
NDC Code(s):
70771-1385-0,
70771-1385-1,
70771-1385-2,
70771-1385-4, view more70771-1386-0, 70771-1386-1, 70771-1386-2, 70771-1386-4, 70771-1387-0, 70771-1387-1, 70771-1387-2, 70771-1387-4, 70771-1388-0, 70771-1388-1, 70771-1388-2, 70771-1388-4, 70771-1389-0, 70771-1389-1, 70771-1389-2, 70771-1389-4
- Packager: Zydus Lifesciences Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 3, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- MEDICATION GUIDE
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Doxepin Hydrochloride Capsules USP, 10 mg
Rx only
100 Capsules

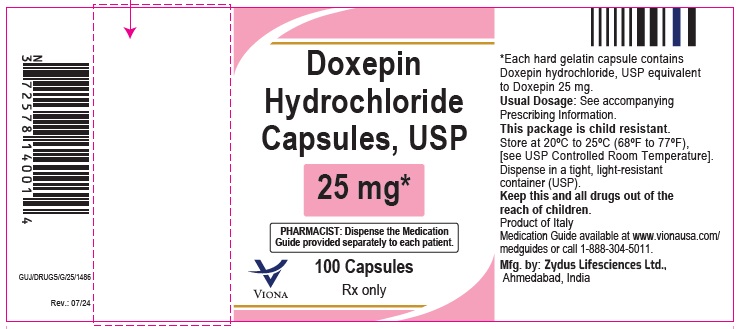
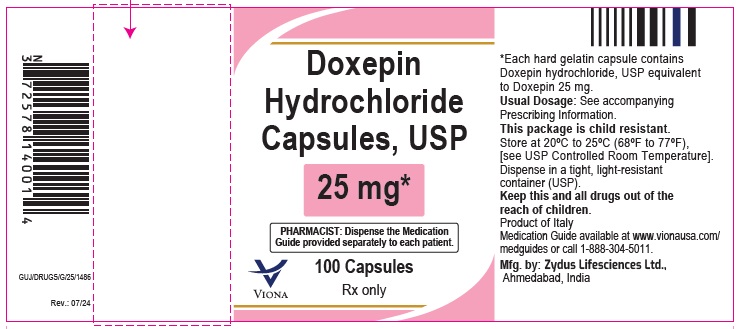
Doxepin Hydrochloride Capsules USP, 25 mg
Rx only
100 Capsules
Doxepin Hydrochloride Capsules USP, 50 mg
Rx only
100 Capsules

Doxepin Hydrochloride Capsules USP, 75 mg
Rx only
100 Capsules

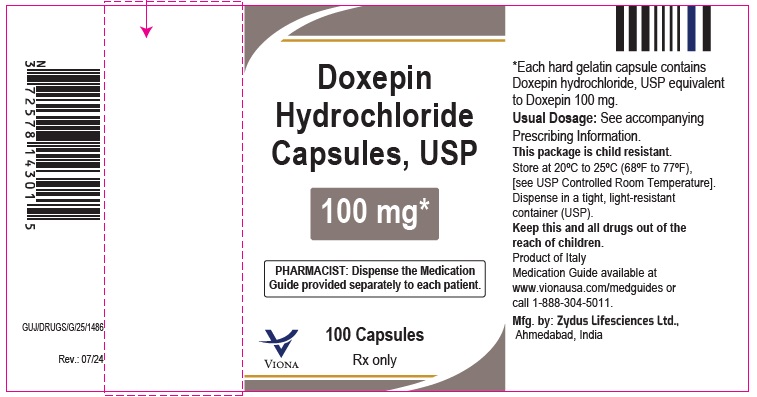
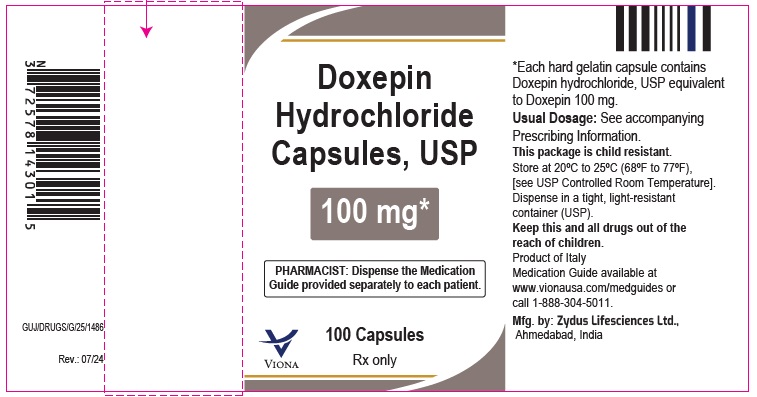
Doxepin Hydrochloride Capsules USP, 100 mg
Rx only
100 Capsules
-
INGREDIENTS AND APPEARANCE
DOXEPIN HYDROCHLORIDE
doxepin hydrochloride capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70771-1385 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOXEPIN HYDROCHLORIDE (UNII: 3U9A0FE9N5) (DOXEPIN - UNII:5ASJ6HUZ7D) DOXEPIN 10 mg Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) AMMONIA (UNII: 5138Q19F1X) BUTYL ALCOHOL (UNII: 8PJ61P6TS3) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) FERROSOFERRIC OXIDE (UNII: XM0M87F357) GELATIN (UNII: 2G86QN327L) ISOPROPYL ALCOHOL (UNII: ND2M416302) MAGNESIUM STEARATE (UNII: 70097M6I30) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHELLAC (UNII: 46N107B71O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM LAURYL SULFATE (UNII: 368GB5141J) STARCH, CORN (UNII: O8232NY3SJ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) WATER (UNII: 059QF0KO0R) Product Characteristics Color WHITE (opaque white cap) , WHITE (opaque white body) Score no score Shape CAPSULE Size 14mm Flavor Imprint Code 1296 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70771-1385-1 100 in 1 BOTTLE; Type 0: Not a Combination Product 03/01/2024 2 NDC:70771-1385-0 1000 in 1 BOTTLE; Type 0: Not a Combination Product 03/01/2024 3 NDC:70771-1385-4 10 in 1 CARTON 03/01/2024 3 NDC:70771-1385-2 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA210700 03/01/2024 DOXEPIN HYDROCHLORIDE
doxepin hydrochloride capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70771-1386 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOXEPIN HYDROCHLORIDE (UNII: 3U9A0FE9N5) (DOXEPIN - UNII:5ASJ6HUZ7D) DOXEPIN 25 mg Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) AMMONIA (UNII: 5138Q19F1X) BUTYL ALCOHOL (UNII: 8PJ61P6TS3) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) GELATIN (UNII: 2G86QN327L) ISOPROPYL ALCOHOL (UNII: ND2M416302) MAGNESIUM STEARATE (UNII: 70097M6I30) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHELLAC (UNII: 46N107B71O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM LAURYL SULFATE (UNII: 368GB5141J) STARCH, CORN (UNII: O8232NY3SJ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) WATER (UNII: 059QF0KO0R) Product Characteristics Color YELLOW (opaque yellow cap) , WHITE (opaque white body) Score no score Shape CAPSULE Size 16mm Flavor Imprint Code 1297 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70771-1386-1 100 in 1 BOTTLE; Type 0: Not a Combination Product 03/01/2024 2 NDC:70771-1386-4 10 in 1 CARTON 03/01/2024 2 NDC:70771-1386-2 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 3 NDC:70771-1386-0 1000 in 1 BOTTLE; Type 0: Not a Combination Product 03/01/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA210700 03/01/2024 DOXEPIN HYDROCHLORIDE
doxepin hydrochloride capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70771-1387 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOXEPIN HYDROCHLORIDE (UNII: 3U9A0FE9N5) (DOXEPIN - UNII:5ASJ6HUZ7D) DOXEPIN 50 mg Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) AMMONIA (UNII: 5138Q19F1X) BUTYL ALCOHOL (UNII: 8PJ61P6TS3) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) GELATIN (UNII: 2G86QN327L) ISOPROPYL ALCOHOL (UNII: ND2M416302) MAGNESIUM STEARATE (UNII: 70097M6I30) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHELLAC (UNII: 46N107B71O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM LAURYL SULFATE (UNII: 368GB5141J) STARCH, PREGELATINIZED CORN (UNII: O8232NY3SJ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) WATER (UNII: 059QF0KO0R) Product Characteristics Color YELLOW (opaque yellow cap) , YELLOW (opaque yellow body) Score no score Shape CAPSULE Size 18mm Flavor Imprint Code 1298 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70771-1387-1 100 in 1 BOTTLE; Type 0: Not a Combination Product 03/01/2024 2 NDC:70771-1387-0 1000 in 1 BOTTLE; Type 0: Not a Combination Product 03/01/2024 3 NDC:70771-1387-4 10 in 1 CARTON 03/01/2024 3 NDC:70771-1387-2 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA210700 03/01/2024 DOXEPIN HYDROCHLORIDE
doxepin hydrochloride capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70771-1388 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOXEPIN HYDROCHLORIDE (UNII: 3U9A0FE9N5) (DOXEPIN - UNII:5ASJ6HUZ7D) DOXEPIN 75 mg Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) AMMONIA (UNII: 5138Q19F1X) BUTYL ALCOHOL (UNII: 8PJ61P6TS3) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C GREEN NO. 3 (UNII: 3P3ONR6O1S) FERROSOFERRIC OXIDE (UNII: XM0M87F357) GELATIN (UNII: 2G86QN327L) ISOPROPYL ALCOHOL (UNII: ND2M416302) MAGNESIUM STEARATE (UNII: 70097M6I30) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHELLAC (UNII: 46N107B71O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM LAURYL SULFATE (UNII: 368GB5141J) STARCH, PREGELATINIZED CORN (UNII: O8232NY3SJ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) WATER (UNII: 059QF0KO0R) Product Characteristics Color GREEN (opaque green cap) , GREEN (opaque green body) Score no score Shape CAPSULE Size 18mm Flavor Imprint Code 1299 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70771-1388-1 100 in 1 BOTTLE; Type 0: Not a Combination Product 03/01/2024 2 NDC:70771-1388-0 1000 in 1 BOTTLE; Type 0: Not a Combination Product 03/01/2024 3 NDC:70771-1388-4 10 in 1 CARTON 03/01/2024 3 NDC:70771-1388-2 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA210700 03/01/2024 DOXEPIN HYDROCHLORIDE
doxepin hydrochloride capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70771-1389 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOXEPIN HYDROCHLORIDE (UNII: 3U9A0FE9N5) (DOXEPIN - UNII:5ASJ6HUZ7D) DOXEPIN 100 mg Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) AMMONIA (UNII: 5138Q19F1X) BUTYL ALCOHOL (UNII: 8PJ61P6TS3) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C GREEN NO. 3 (UNII: 3P3ONR6O1S) FERROSOFERRIC OXIDE (UNII: XM0M87F357) GELATIN (UNII: 2G86QN327L) ISOPROPYL ALCOHOL (UNII: ND2M416302) MAGNESIUM STEARATE (UNII: 70097M6I30) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHELLAC (UNII: 46N107B71O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM LAURYL SULFATE (UNII: 368GB5141J) STARCH, PREGELATINIZED CORN (UNII: O8232NY3SJ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) WATER (UNII: 059QF0KO0R) Product Characteristics Color GREEN (opaque green cap) , WHITE (opaque white body) Score no score Shape CAPSULE Size 19mm Flavor Imprint Code 1300 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70771-1389-1 100 in 1 BOTTLE; Type 0: Not a Combination Product 03/01/2024 2 NDC:70771-1389-0 1000 in 1 BOTTLE; Type 0: Not a Combination Product 03/01/2024 3 NDC:70771-1389-4 10 in 1 CARTON 03/01/2024 3 NDC:70771-1389-2 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA210700 03/01/2024 Labeler - Zydus Lifesciences Limited (918596198) Establishment Name Address ID/FEI Business Operations Zydus Lifesciences Limited 918596198 ANALYSIS(70771-1385, 70771-1386, 70771-1387, 70771-1388, 70771-1389) , MANUFACTURE(70771-1385, 70771-1386, 70771-1387, 70771-1388, 70771-1389)