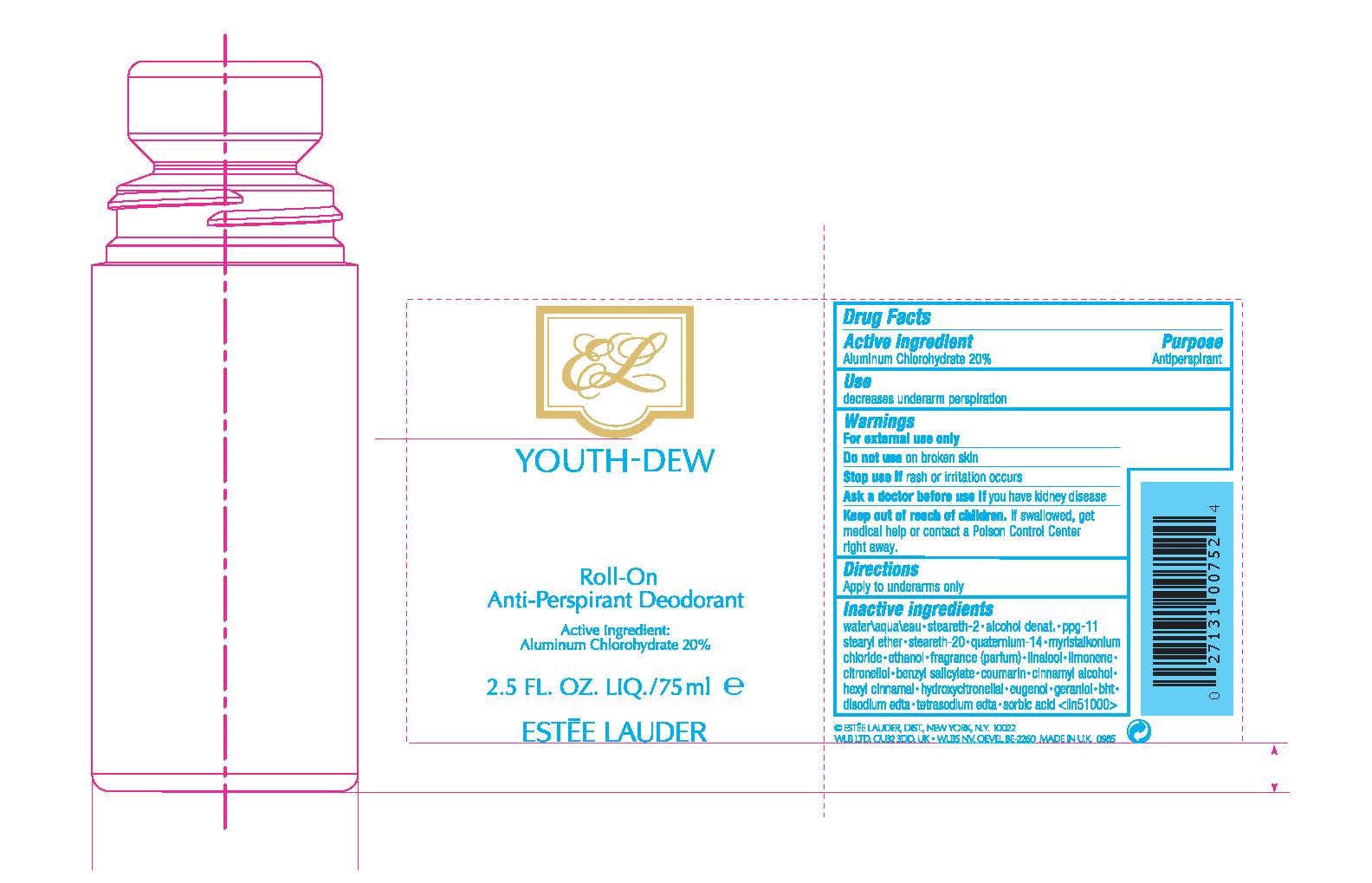
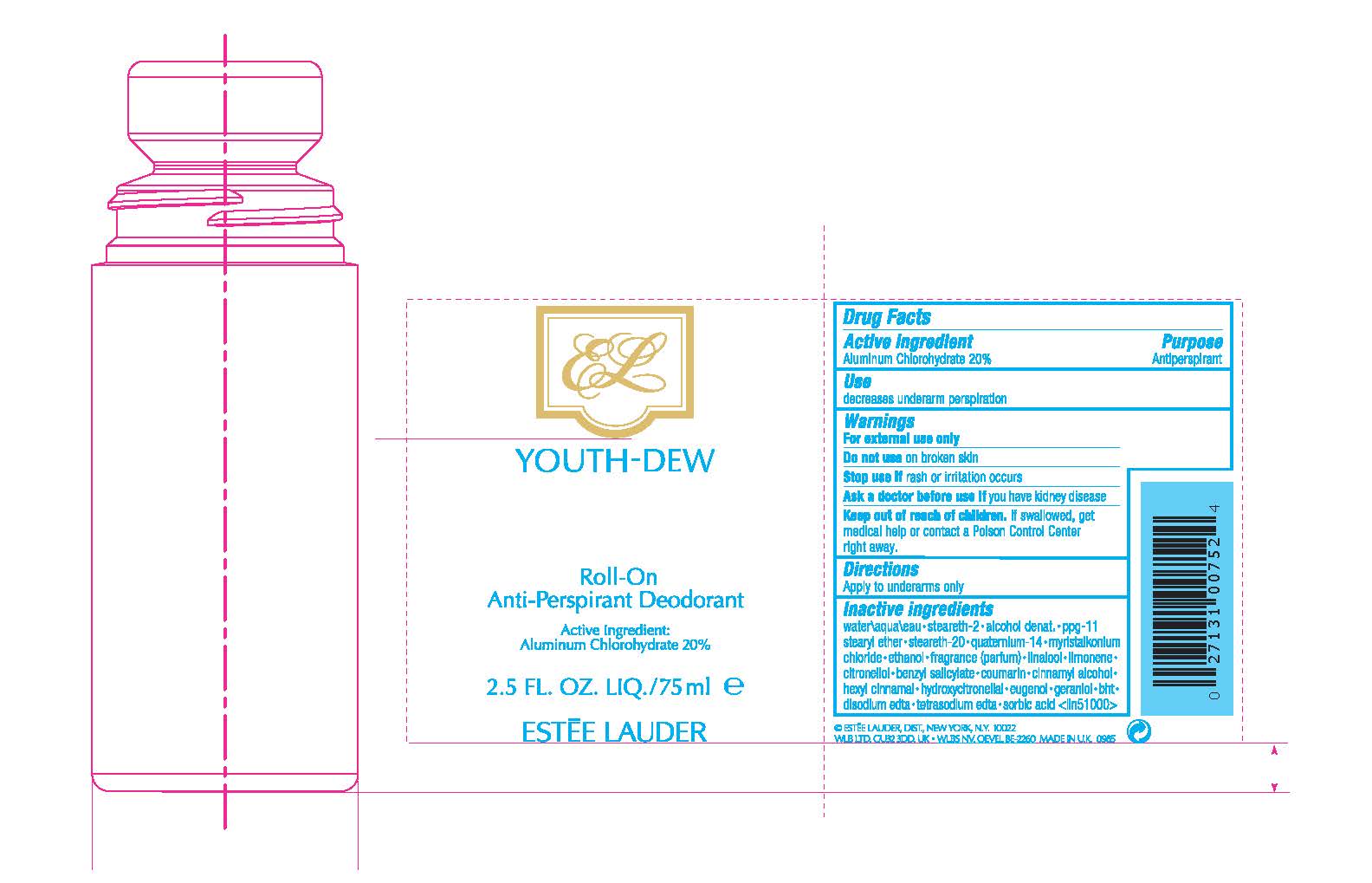
Label: YOUTH DEW ROLL-ON ANTIPERSPIRANT DEODORANT- aluminum chlorohydrate liquid
- NDC Code(s): 11559-001-01
- Packager: ESTEE LAUDER INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated July 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
-
Inactive ingredients
water\aqua\eau•steareth-2•alcohol denat.•ppg-11 stearyl ether•steareth-20•youth-dew fragrance (parfum)•myristalkonium chloride•quaternium-14•benzyl salicylate•linalool•hydroxycitronellal•geraniol•citronellol•eugenol•limonene•cinnamyl alcohol•coumarin•hexyl cinnamal•trisodium edta•sorbic acid <iln37908>
- PRINCIPAL DISPLAY PANEL - 75 ml Bottle Label
-
INGREDIENTS AND APPEARANCE
YOUTH DEW ROLL-ON ANTIPERSPIRANT DEODORANT
aluminum chlorohydrate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11559-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM CHLOROHYDRATE (UNII: HPN8MZW13M) (ALUMINUM CHLOROHYDRATE - UNII:HPN8MZW13M) ALUMINUM CHLOROHYDRATE 200 mg in 1 mL Inactive Ingredients Ingredient Name Strength LINALOOL, (+)- (UNII: F4VNO44C09) HYDROXYCITRONELLAL (UNII: 8SQ0VA4YUR) GERANIOL (UNII: L837108USY) CITRONELLOL ACETATE, (R)- (UNII: 9X45FJL446) EUGENOL (UNII: 3T8H1794QW) LIMONENE, (+)- (UNII: GFD7C86Q1W) CINNAMYL ALCOHOL (UNII: SS8YOP444F) COUMARIN (UNII: A4VZ22K1WT) .ALPHA.-HEXYLCINNAMALDEHYDE (UNII: 7X6O37OK2I) EDETATE TRISODIUM (UNII: 420IP921MB) SORBIC ACID (UNII: X045WJ989B) WATER (UNII: 059QF0KO0R) STEARETH-2 (UNII: V56DFE46J5) ALCOHOL (UNII: 3K9958V90M) POLYPROPYLENE GLYCOL 11 STEARYL ETHER (UNII: S4G2J0Y0LG) STEARETH-20 (UNII: L0Q8IK9E08) FRAGRANCE LAVENDER & CHIA F-153480 (UNII: SXS9CO2TZK) MYRISTALKONIUM CHLORIDE (UNII: 0W255OL75T) QUATERNIUM-14 (UNII: ZGE94G6AGI) BENZYL SALICYLATE (UNII: WAO5MNK9TU) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11559-001-01 75 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product 12/02/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M019 12/02/2020 Labeler - ESTEE LAUDER INC (005914387) Registrant - Estee Lauder Companies Inc. (790802086) Establishment Name Address ID/FEI Business Operations Whitman Laboratories Ltd. 216866277 manufacture(11559-001) , pack(11559-001) , label(11559-001)