Label: CANNASERENE HOT AND COLD CBD CREAM- hot and cold cream cream
- NDC Code(s): 74787-1801-0, 74787-1801-2, 74787-1801-3
- Packager: BioSerene Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated May 10, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
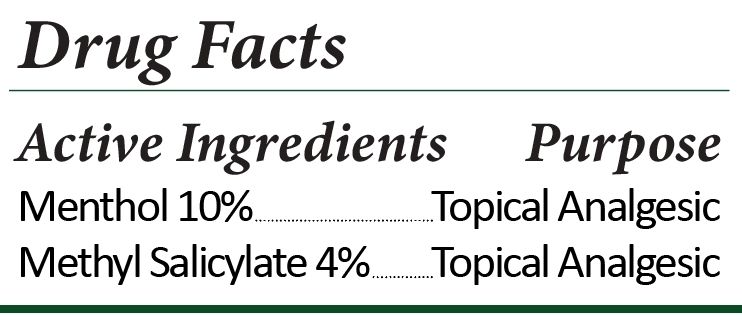
- Active Ingredients
- Uses
- Purpose
- Directions
-
Warnings
FOR EXTERNAL USE ONLY.
Flammable. Keep away from fire or flame.
Allergy Alert. If prone to allergic reaction from aspirin or salicylates, consult doctor before use.
When Using This Product
- Use only as directed
- Avoid contact with eyes or mucous membranes
- Do not bandage tightly
- Storage and Handling
-
Inactive Ingredients
Purified Water, Menthol, Gaultheria Procumbens (Wintergreen) Leaf Oil, Fractionated Coconut Oil, Polysorbate 80, Sorbitan Stearate, Cetearyl Alcohol, Isopropyl Myristate, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Cannabidiol, Dimethyl Isosorbide, Oenothera Biennis (EveningPrimrose) Oil, Tocopherol Acetate (VitaminE), Propylene Glycol, Diazolidinyl Urea, Iodopropynyl Butylcarbamate
- Labeling
-
INGREDIENTS AND APPEARANCE
CANNASERENE HOT AND COLD CBD CREAM
hot and cold cream creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:74787-1801 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 4 g in 100 mL MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 10 g in 100 mL Inactive Ingredients Ingredient Name Strength DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) POLYSORBATE 80 (UNII: 6OZP39ZG8H) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (100000 MPA.S AT 1.5%) (UNII: 86FQE96TZ4) CANNABIDIOL (UNII: 19GBJ60SN5) DIMETHYL ISOSORBIDE (UNII: SA6A6V432S) EVENING PRIMROSE OIL (UNII: 3Q9L08K71N) SORBITAN MONOSTEARATE (UNII: NVZ4I0H58X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:74787-1801-3 100 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 06/14/2022 01/20/2025 2 NDC:74787-1801-2 50 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 06/14/2022 01/20/2025 3 NDC:74787-1801-0 10 mL in 1 TUBE; Type 0: Not a Combination Product 06/14/2022 01/20/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 06/14/2022 01/20/2025 Labeler - BioSerene Inc. (117473724) Establishment Name Address ID/FEI Business Operations BioSerene Inc. 117473724 manufacture(74787-1801)