Label: ANTIMICROBIAL HAND SANITIZER- alcohol gel
-
NDC Code(s):
51811-300-20,
51811-300-21,
51811-300-25,
51811-300-31, view more51811-300-40, 51811-300-41, 51811-300-44, 51811-300-45, 51811-300-50, 51811-300-51, 51811-300-52
- Packager: HPC Ventures, LLC
- This is a repackaged label.
- Source NDC Code(s): 61010-4111
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 27, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
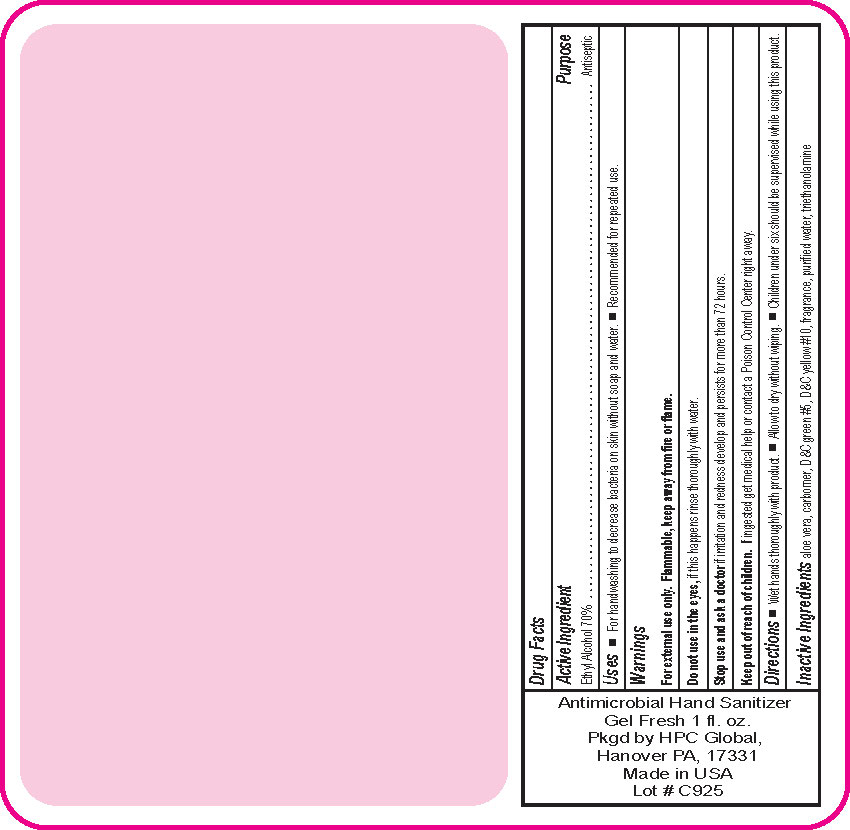
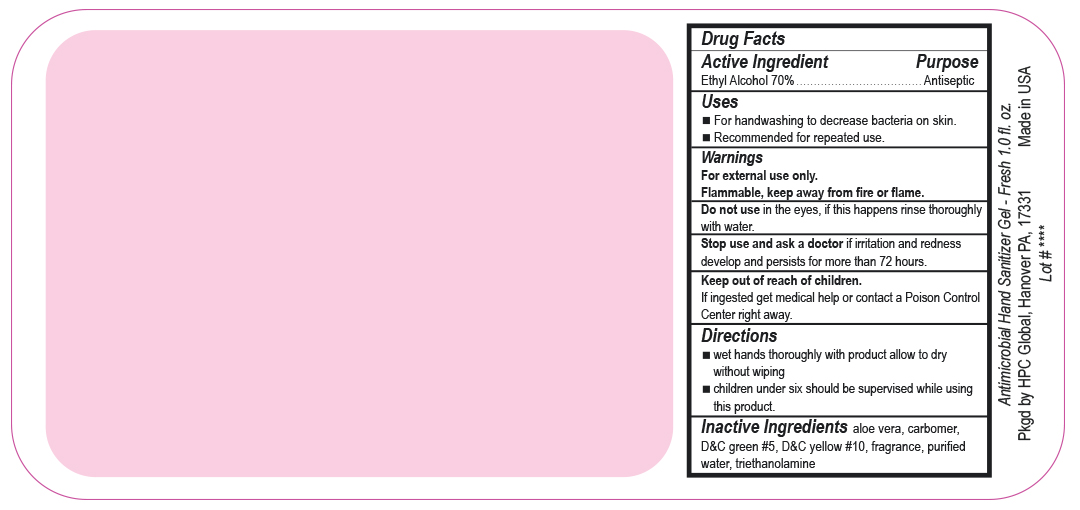
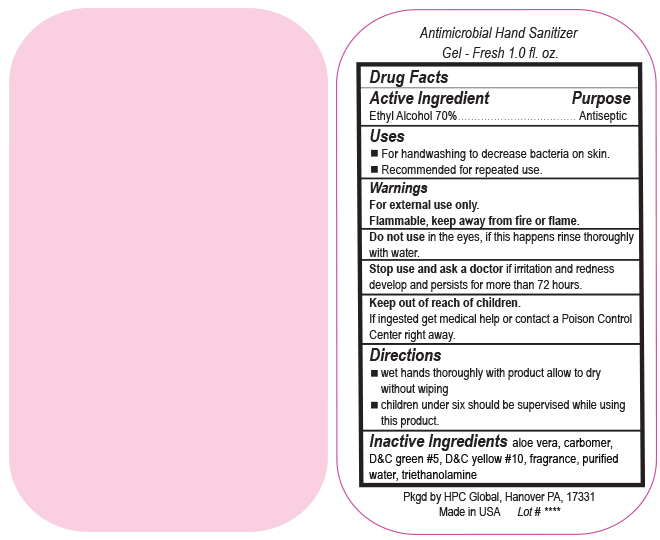
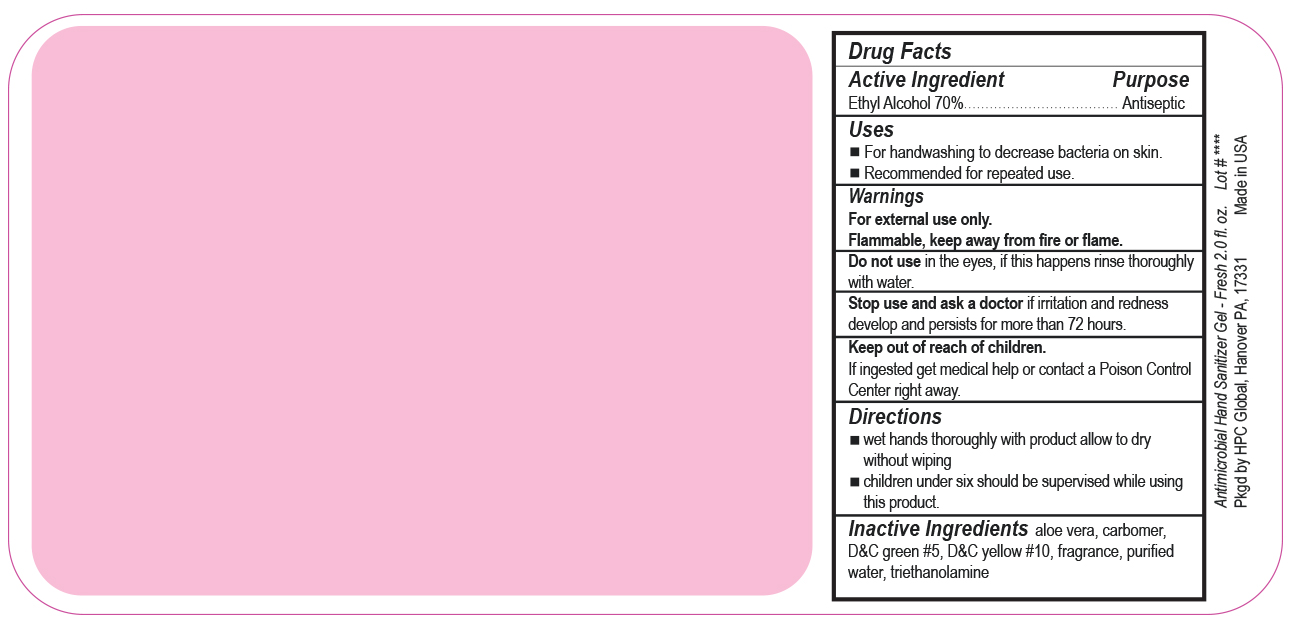
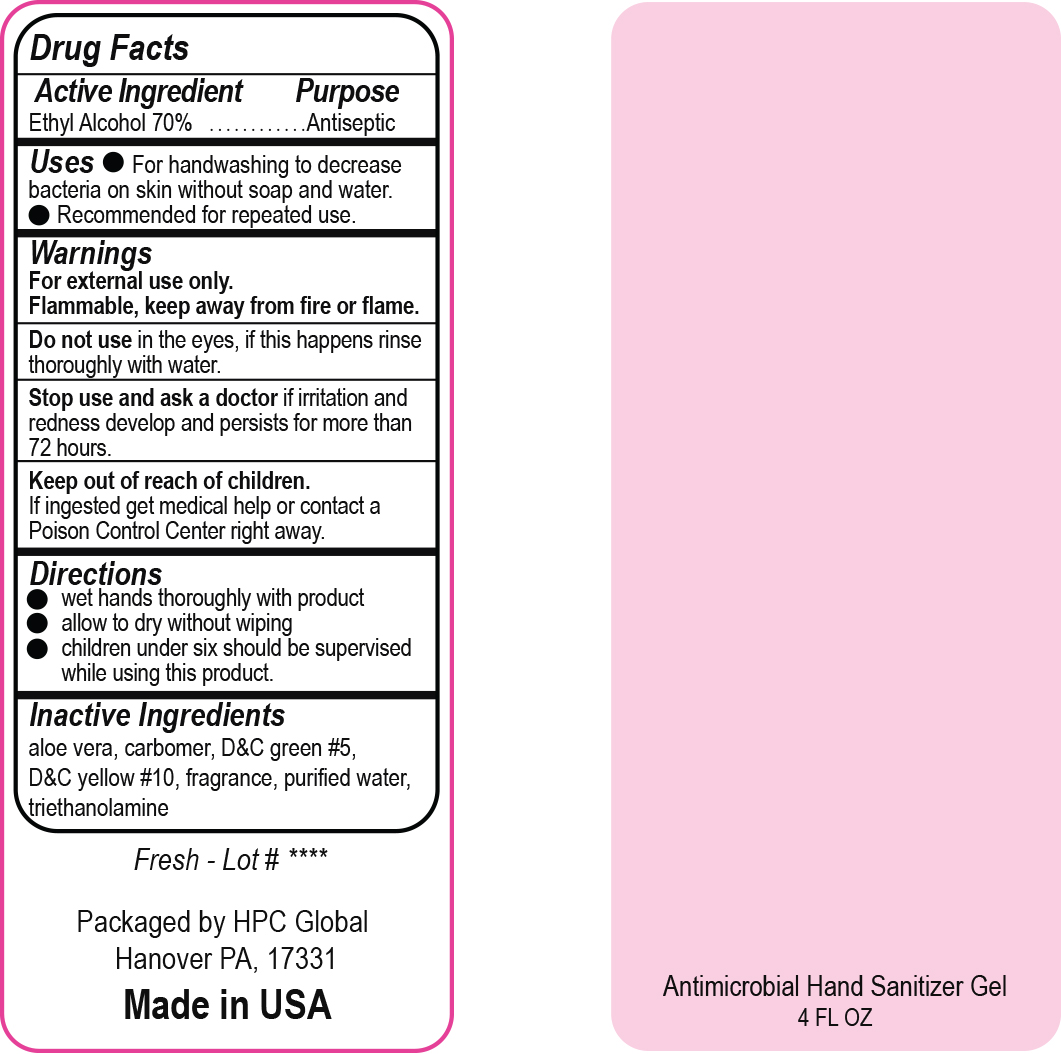
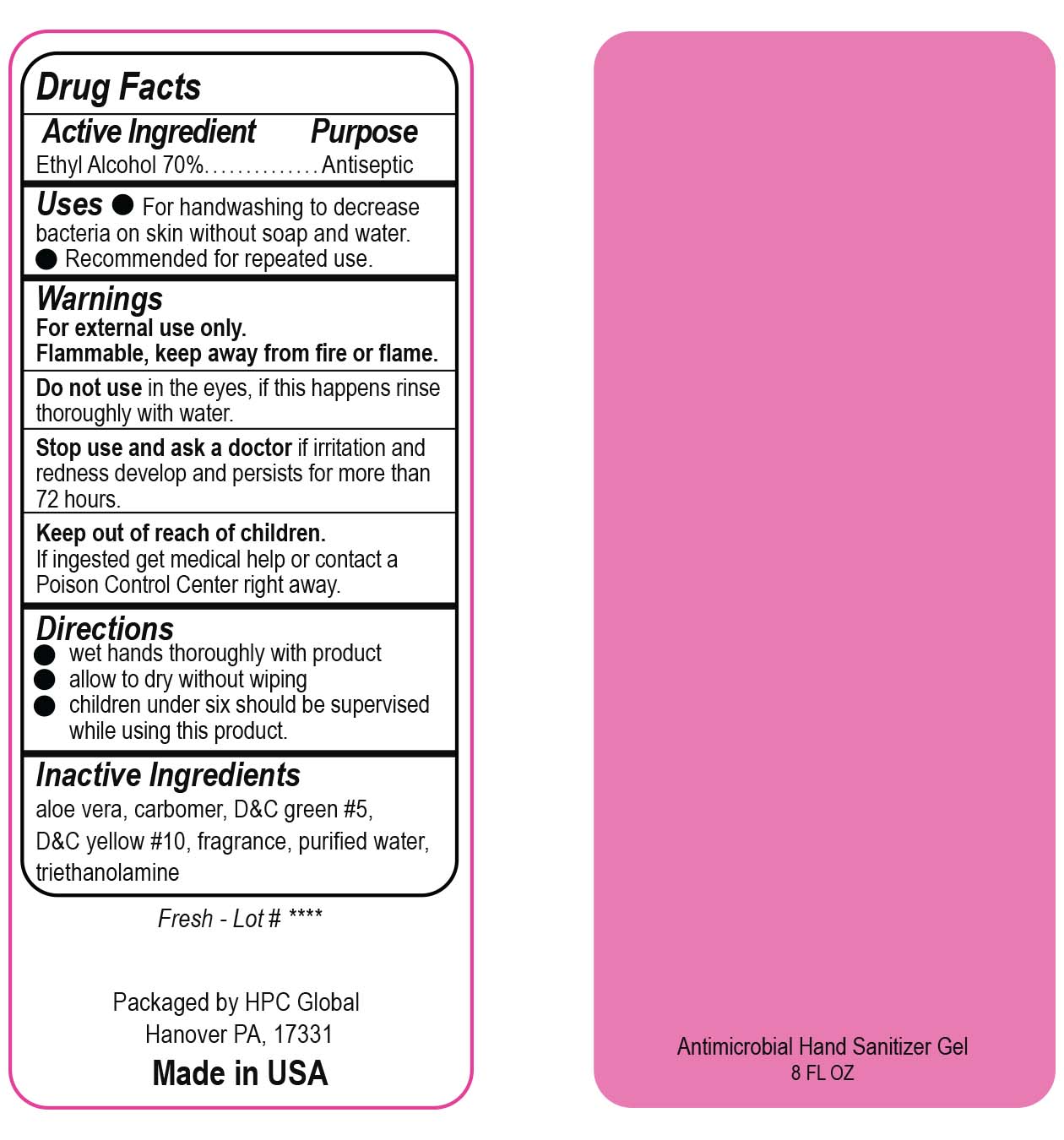
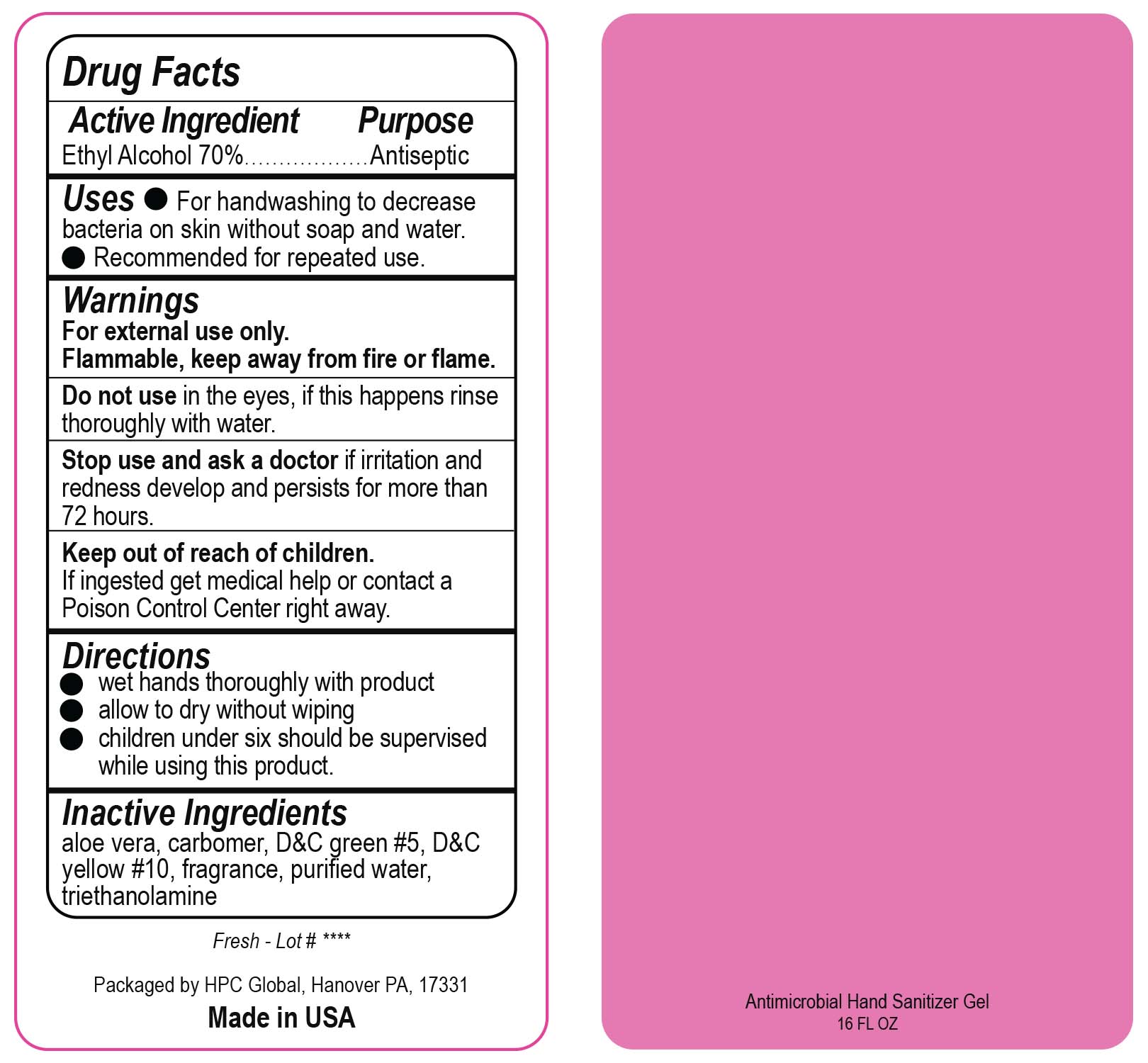
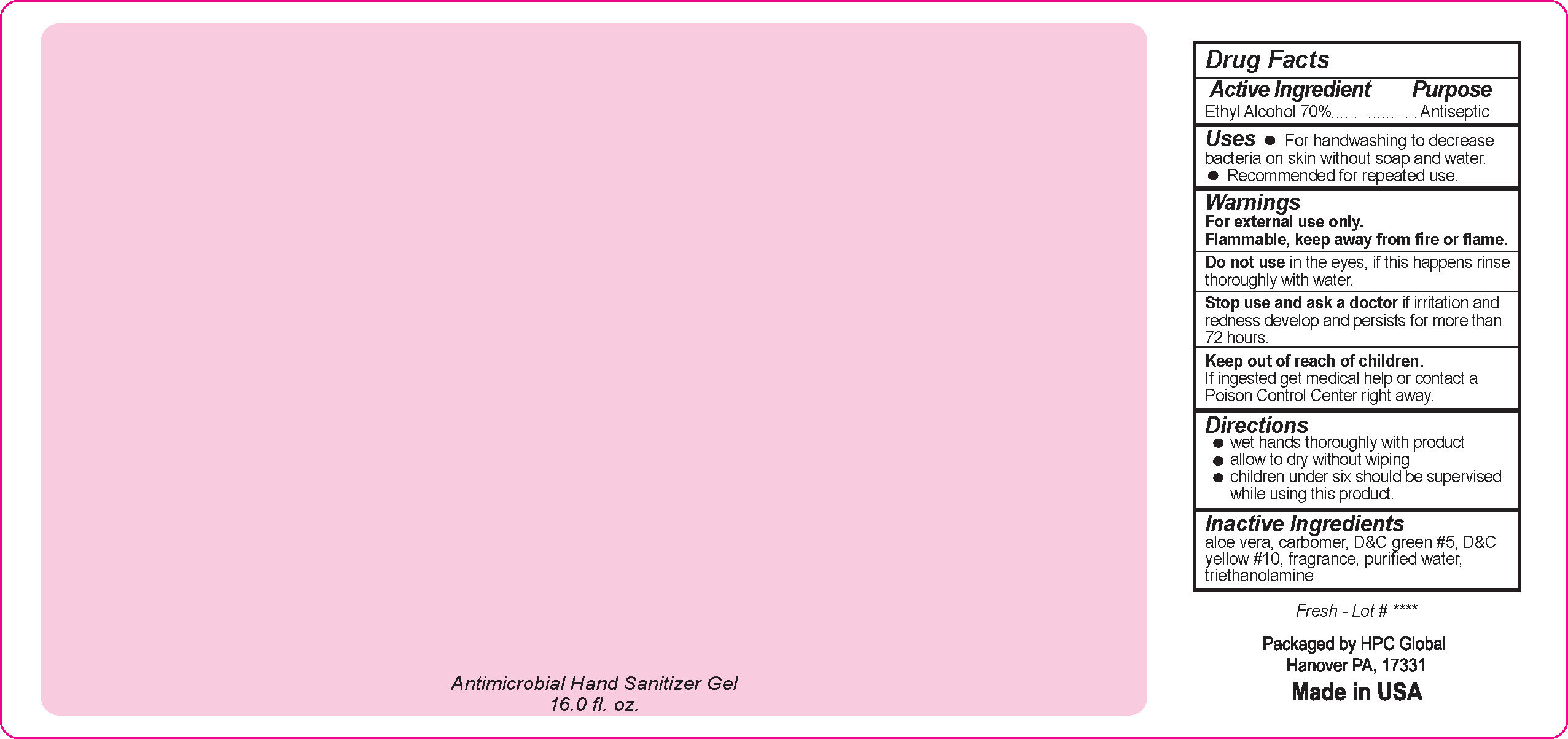
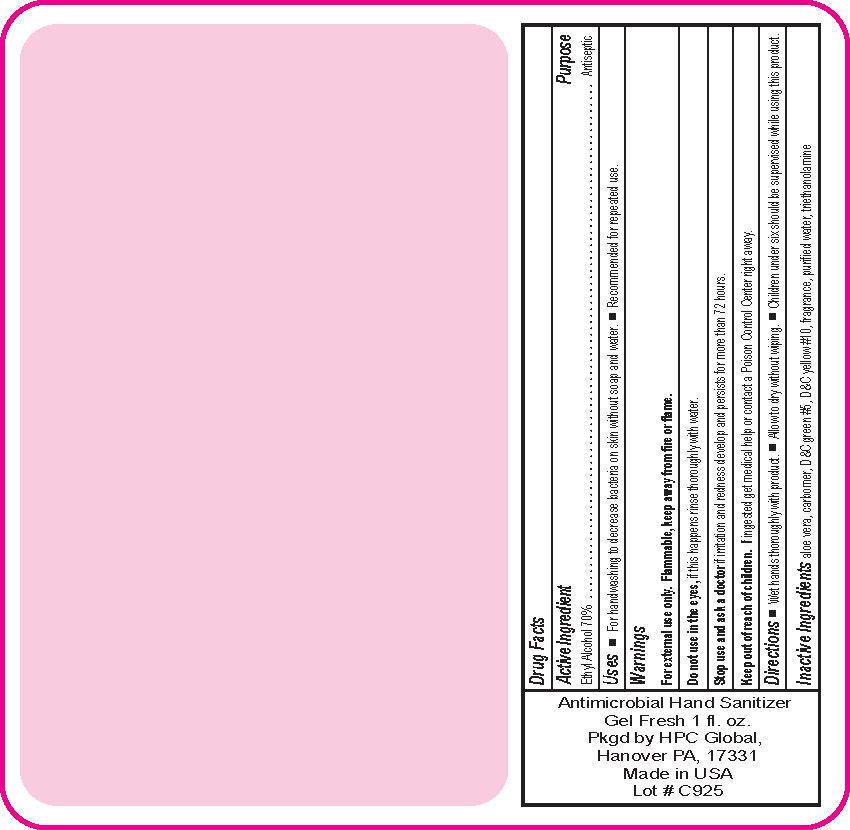
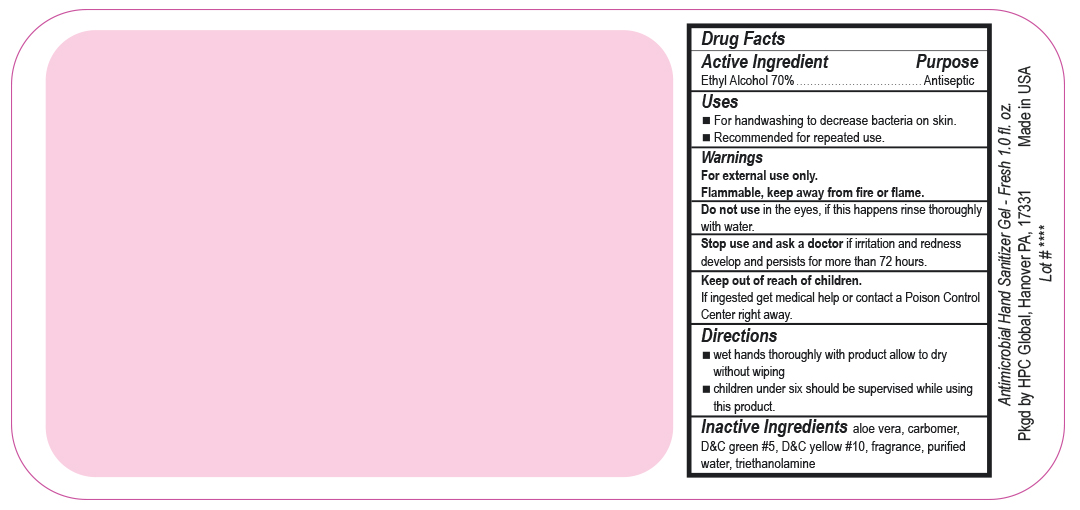
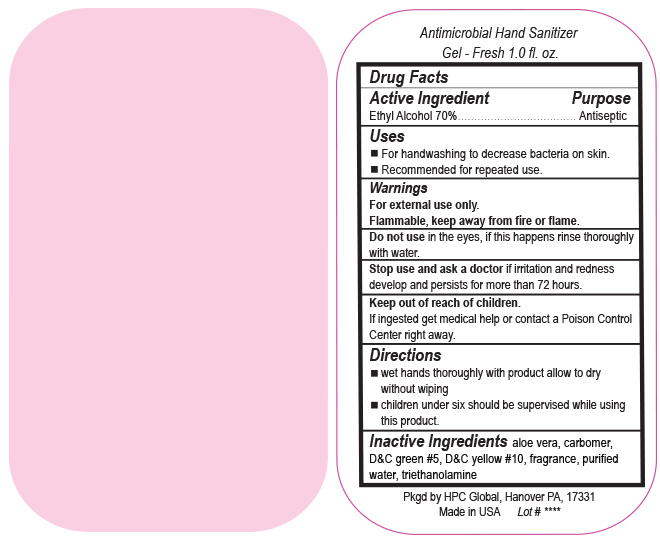
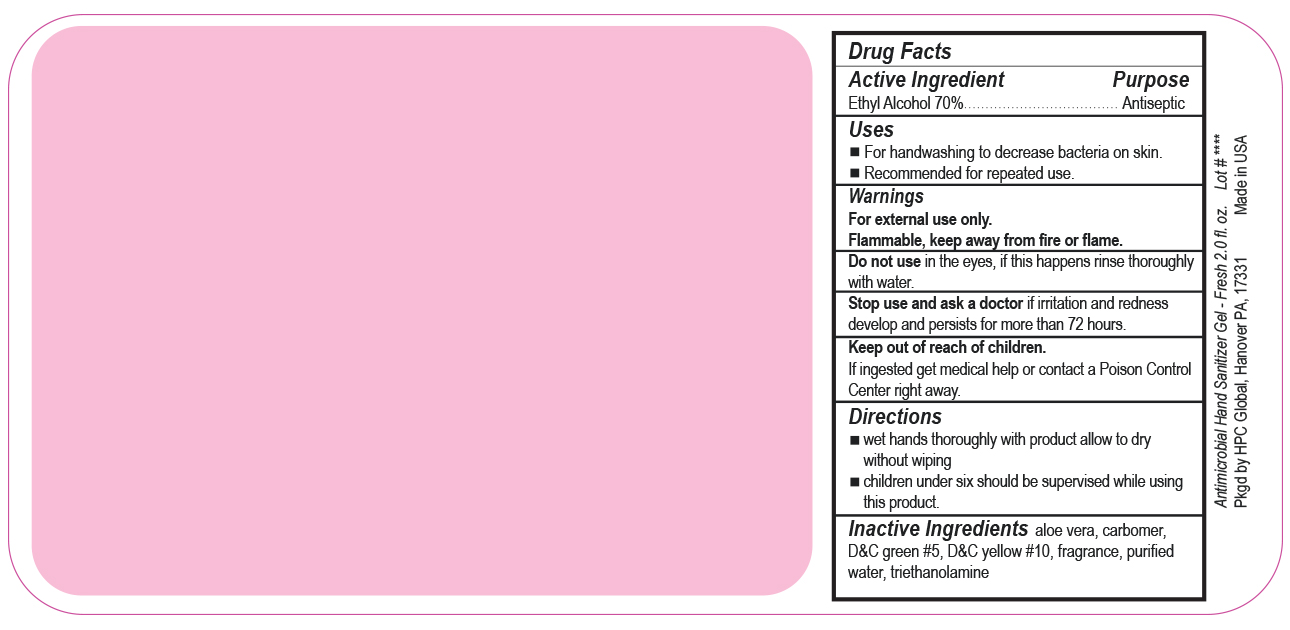
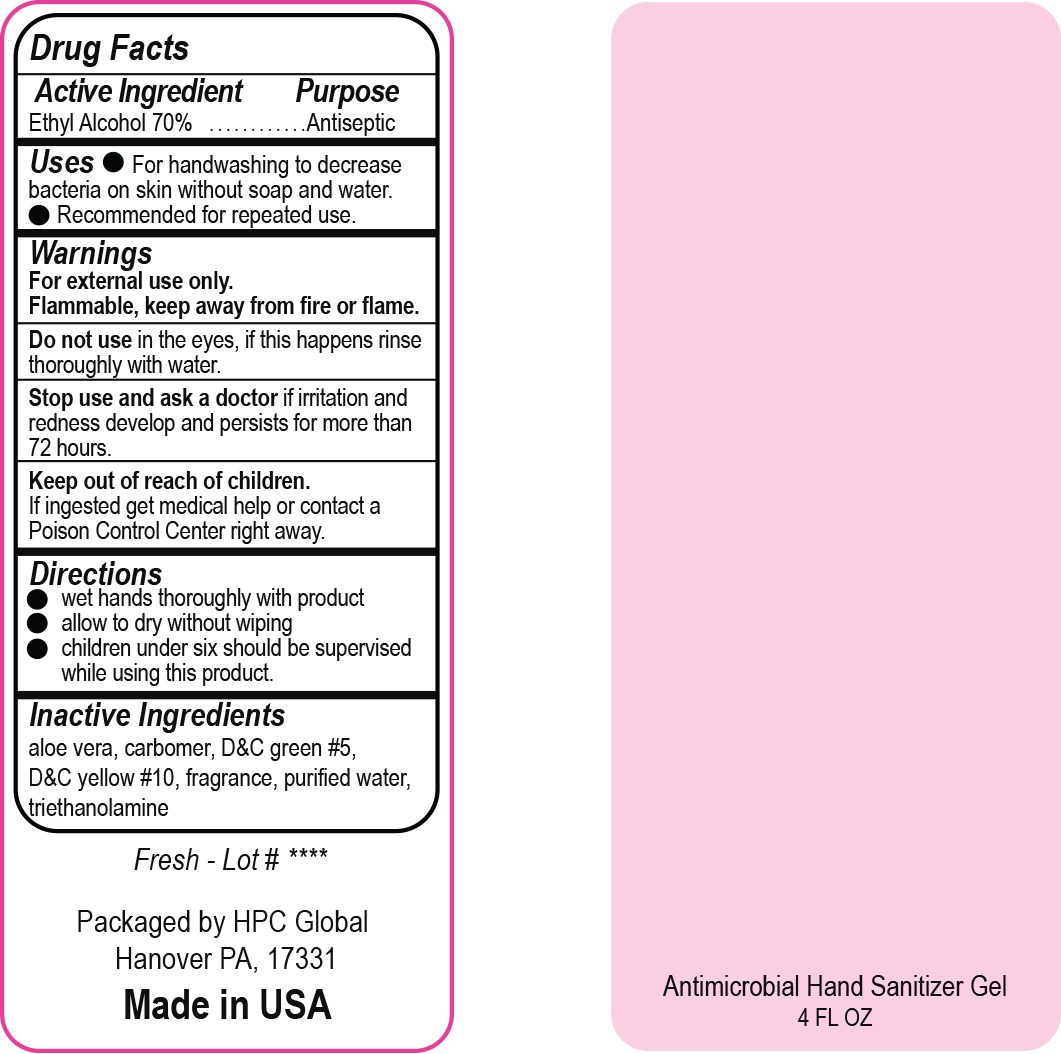
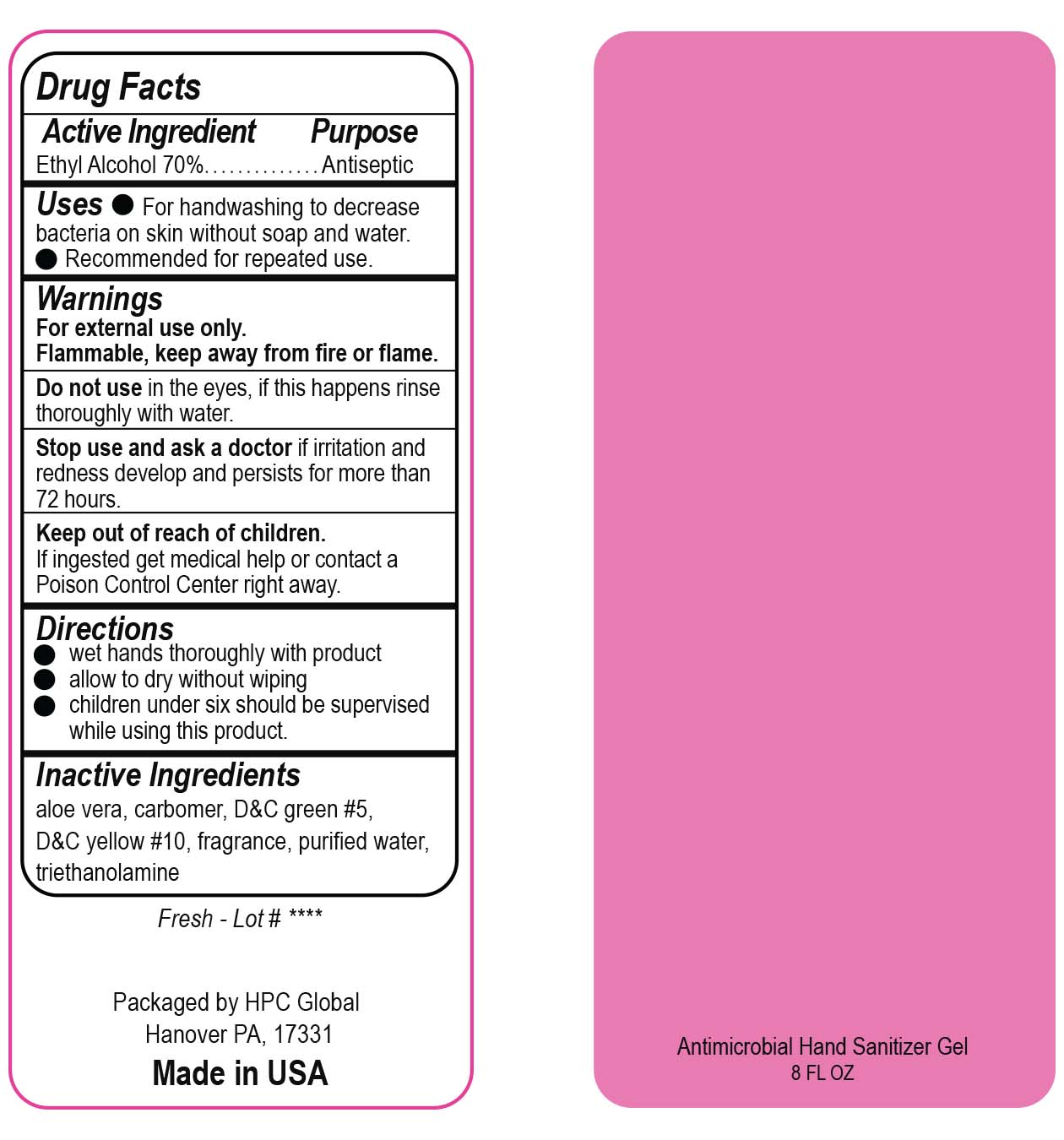
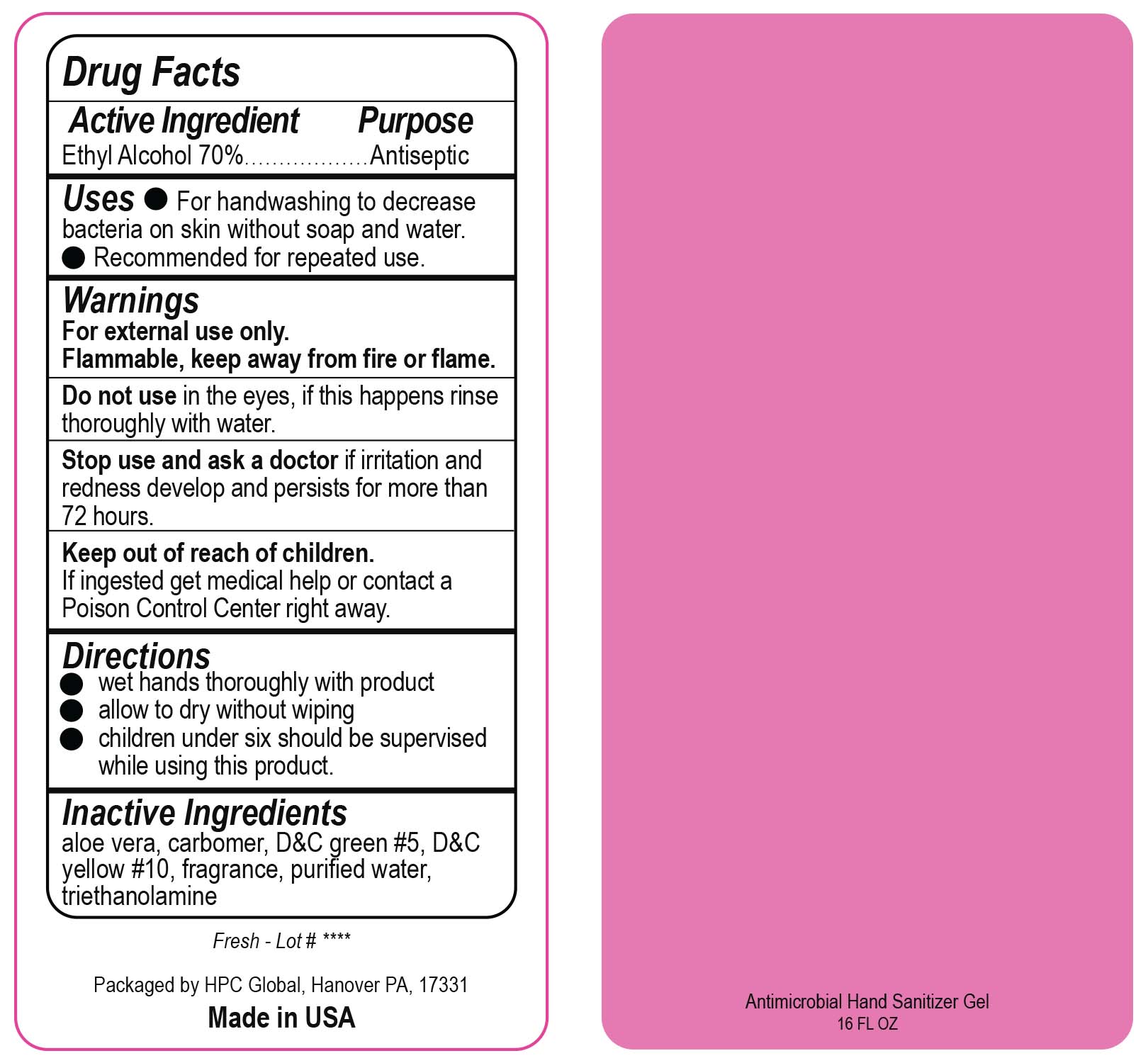
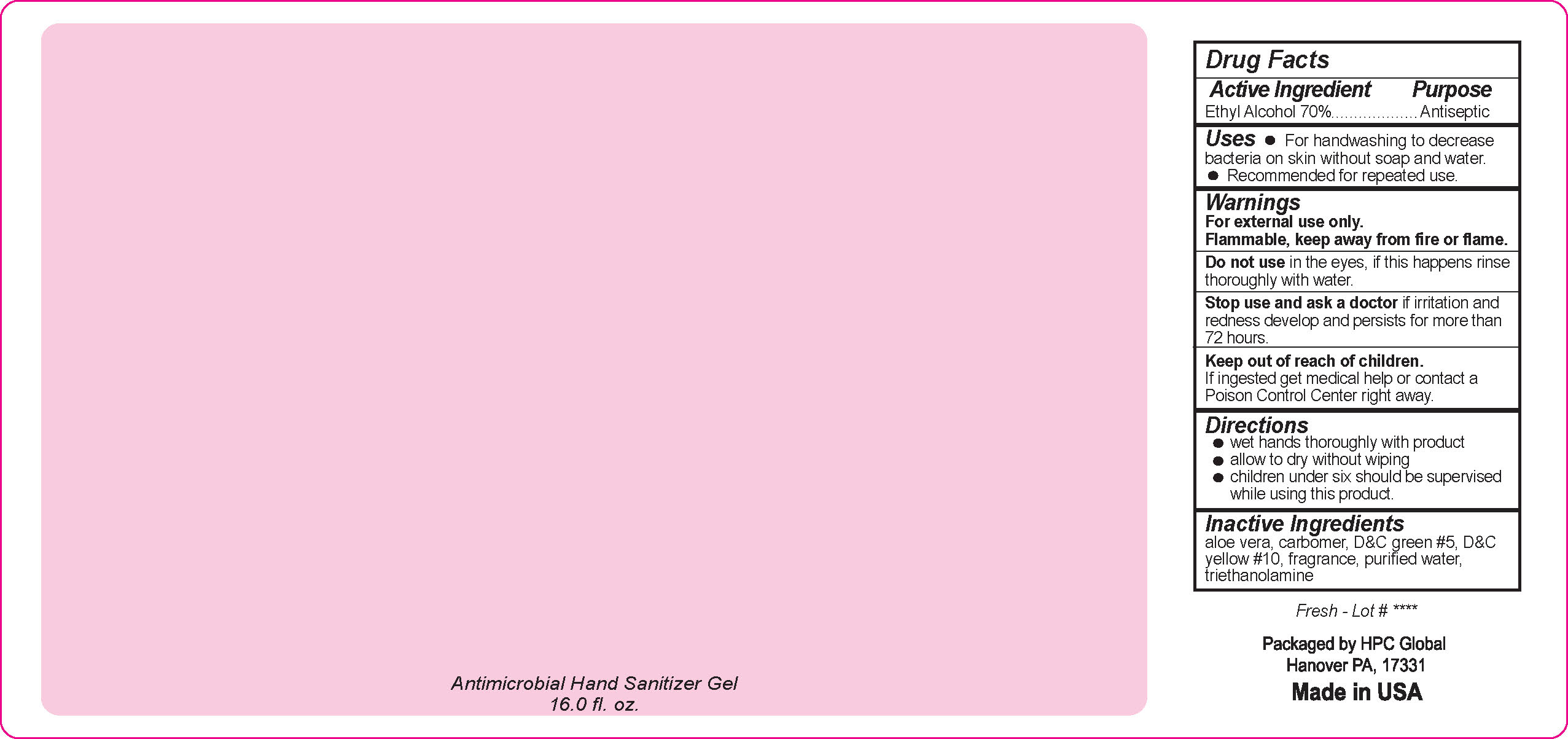
- Drug Facts
- Uses
- Warnings
- DO NOT USE
- ASK DOCTOR
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Inactive ingredients
- SPL UNCLASSIFIED SECTION
- NDC 51811-300-20
- NDC 51811-300-21
- NDC 51811-300-25
- NDC 51811-300-31
- NDC 51811-300-40 and 51811-300-41
- NDC 51811-300-44 and 51811-300-45
- NDC 51811-300-50
- NDC 51811-300-51 and 51811-300-52
-
INGREDIENTS AND APPEARANCE
ANTIMICROBIAL HAND SANITIZER
alcohol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51811-300(NDC:61010-4111) Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 0.7 g in 1 mL Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) WATER (UNII: 059QF0KO0R) D&C GREEN NO. 5 (UNII: 8J6RDU8L9X) TROLAMINE (UNII: 9O3K93S3TK) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51811-300-20 29 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 10/01/2020 2 NDC:51811-300-21 29 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/20/2010 3 NDC:51811-300-25 29 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/20/2010 4 NDC:51811-300-31 59 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/20/2010 5 NDC:51811-300-40 118 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/20/2010 6 NDC:51811-300-41 118 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 12/20/2010 7 NDC:51811-300-44 236 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/20/2010 8 NDC:51811-300-45 236 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 12/20/2010 9 NDC:51811-300-50 473 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 12/20/2010 10 NDC:51811-300-51 473 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 10/01/2020 11 NDC:51811-300-52 473 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 10/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 12/20/2010 Labeler - HPC Ventures, LLC (097538103)