Label: AUVELITY- dextromethorphan hydrobromide, bupropion hydrochloride tablet, multilayer, extended release
- NDC Code(s): 81968-045-14, 81968-045-30, 81968-045-31, 81968-045-60
- Packager: Axsome Therapeutics, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated September 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use AUVELITY safely and effectively. See full prescribing information for AUVELITY. AUVELITY® (dextromethorphan hydrobromide and ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: SUICIDAL THOUGHTS AND BEHAVIORS
Antidepressants increased the risk of suicidal thoughts and behaviors in pediatric and young adult patients in short-term studies. Closely monitor all antidepressant-treated patients for clinical worsening, and emergence of suicidal thoughts and behaviors [see Warnings and Precautions (5.1)]. AUVELITY is not approved for use in pediatric patients [see Use in Specific Populations (8.4)].
Close -
1 INDICATIONS AND USAGEAUVELITY is indicated for the treatment of major depressive disorder (MDD) in adults.
-
2 DOSAGE AND ADMINISTRATION2.1 Important Recommendations Prior to Initiating and During Treatment with AUVELITY - Prior to initiating and during treatment with AUVELITY: assess blood pressure and monitor periodically ...
-
3 DOSAGE FORMS AND STRENGTHSAUVELITY extended-release tablets contain 45 mg dextromethorphan hydrobromide and 105 mg bupropion hydrochloride. The tablets are beige and round with “45/105” debossed on one side.
-
4 CONTRAINDICATIONSAUVELITY is contraindicated in patients: with a seizure disorder [see Warnings and Precautions (5.2)]. with a current or prior diagnosis of bulimia or anorexia nervosa as a higher incidence of ...
-
5 WARNINGS AND PRECAUTIONS5.1 Suicidal Thoughts and Behaviors in Adolescents and Young Adults - In pooled analyses of placebo-controlled trials of antidepressant drugs (SSRIs and other antidepressant classes) that ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed in more detail in other sections of the labeling: Suicidal Thoughts and Behaviors in Adolescents and Young Adults [see Warnings and Precautions ...
-
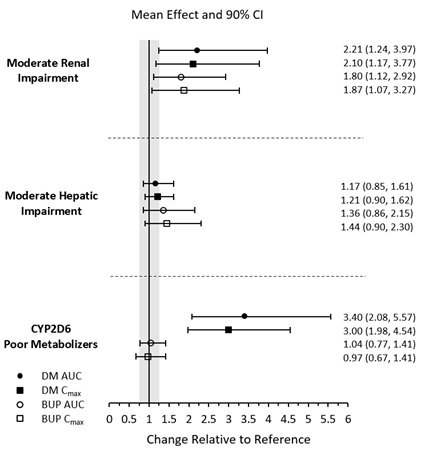
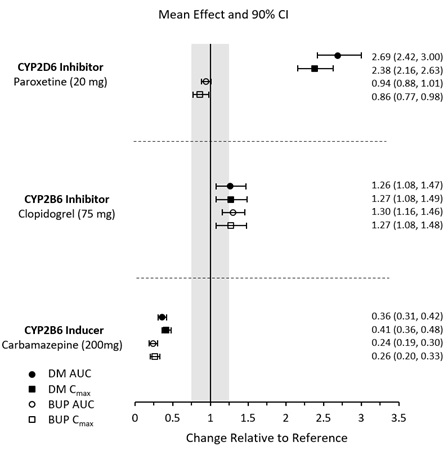
7 DRUG INTERACTIONS7.1 Drugs Having Clinically Important Interactions with AUVELITY - Table 3: Clinically Important Drug Interactions with AUVELITY - Monoamine Oxidase Inhibitors (MAOIs) Clinical ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Exposure Registry - There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to antidepressants, including AUVELITY, during pregnancy ...
-
9 DRUG ABUSE AND DEPENDENCE9.1 Controlled Substance - AUVELITY contains dextromethorphan and bupropion which are not controlled substances. 9.2 Abuse - Abuse is the intentional, non-therapeutic use of a drug, even once ...
-
10 OVERDOSAGEHuman Experience - There is limited clinical study experience regarding human overdosage with AUVELITY. Overdosage information is based on experience with the individual components ...
-
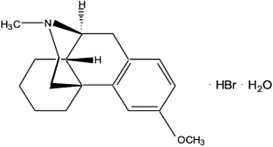
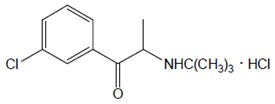
11 DESCRIPTIONAUVELITY is a combination of dextromethorphan hydrobromide, an uncompetitive NMDA receptor antagonist and sigma-1 receptor agonist, and bupropion hydrochloride, an aminoketone and CYP450 2D6 ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Dextromethorphan is an uncompetitive antagonist of the NMDA receptor (an ionotropic glutamate receptor) and a sigma-1 receptor agonist. The mechanism of ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - In a 26-week carcinogenicity study in the Tg.rasH2 transgenic mouse, dextromethorphan at oral doses up to 100 ...
-
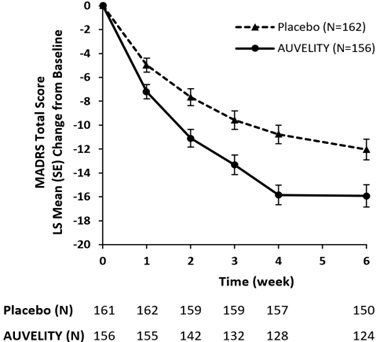
14 CLINICAL STUDIESThe efficacy of AUVELITY for the treatment of MDD in adults was demonstrated in a placebo-controlled clinical study (Study 1, NCT04019704) and confirmatory evidence which included a second study ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGAUVELITY (dextromethorphan hydrobromide and bupropion hydrochloride) extended-release tablets are beige, film-coated, round, bilayer tablets with “45/105” debossed on one side. AUVELITY is ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). Suicidal Thoughts and Behaviors - Advise patients and caregivers to look for the emergence of suicidal ideation and ...
-
MEDICATION GUIDEThis Medication Guide has been approved by the U.S. Food and Drug Administration. AUV-USMG-001.001-20221219 - Issued: 12/2022 - MEDICATION GUIDE - AUVELITY® (aw - VEHL - ah- tee ...
-
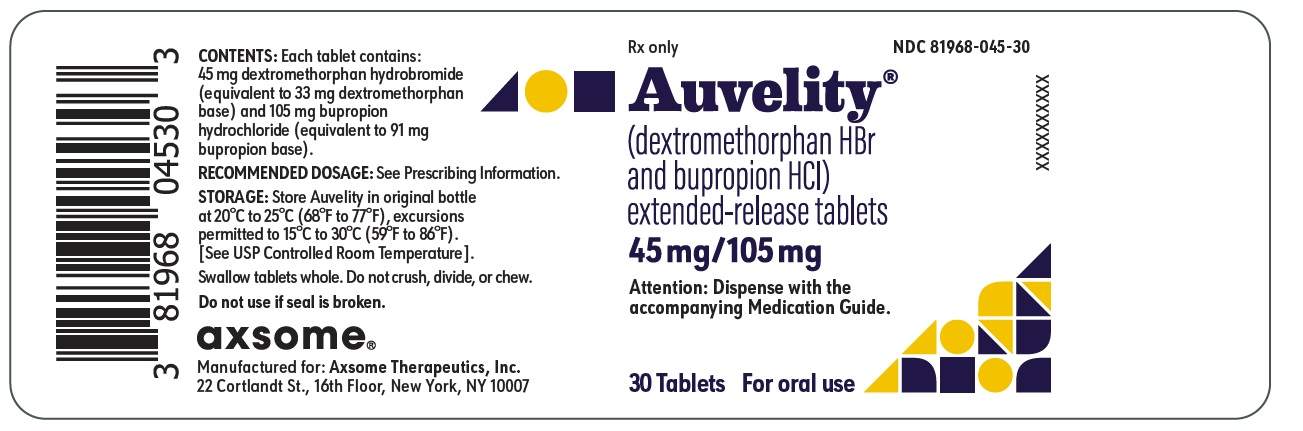
PRINCIPAL DISPLAY PANEL - NDC: 81968-045-30 - 30-count Bottle LabelRx only - NDC 81968-045-30 - Auvelity® (dextromethorphan HBr and bupropion HCl) extended-release tablets - 45mg/105mg - Attention: Dispense with the accompanying Medication Guide. 30 ...
-
PRINCIPAL DISPLAY PANEL - NDC: 81968-045-60 - 60-count Bottle LabelRx only - NDC 81968-045-60 - Auvelity® (dextromethorphan HBr and bupropion HCl) extended-release tablets - 45mg/105mg - Attention: Dispense with the accompanying Medication Guide. 60 ...
-
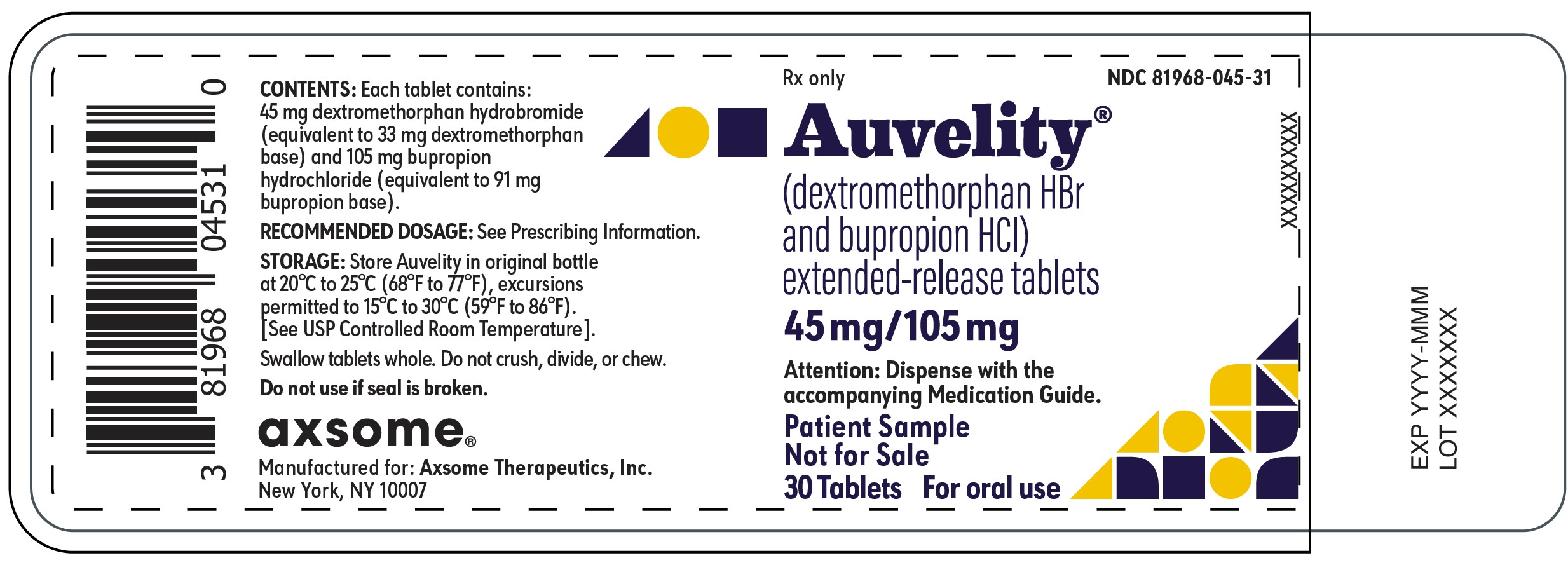
PRINCIPAL DISPLAY PANEL - NDC: 81968-045-31 - 30-count Physician Sample Bottle LabelRx only - NDC 81968-045-31 - Auvelity® (dextromethorphan HBr and bupropion HCl) extended-release tablets - 45mg/105mg - Attention: Dispense with the accompanying Medication Guide. Patient ...
-
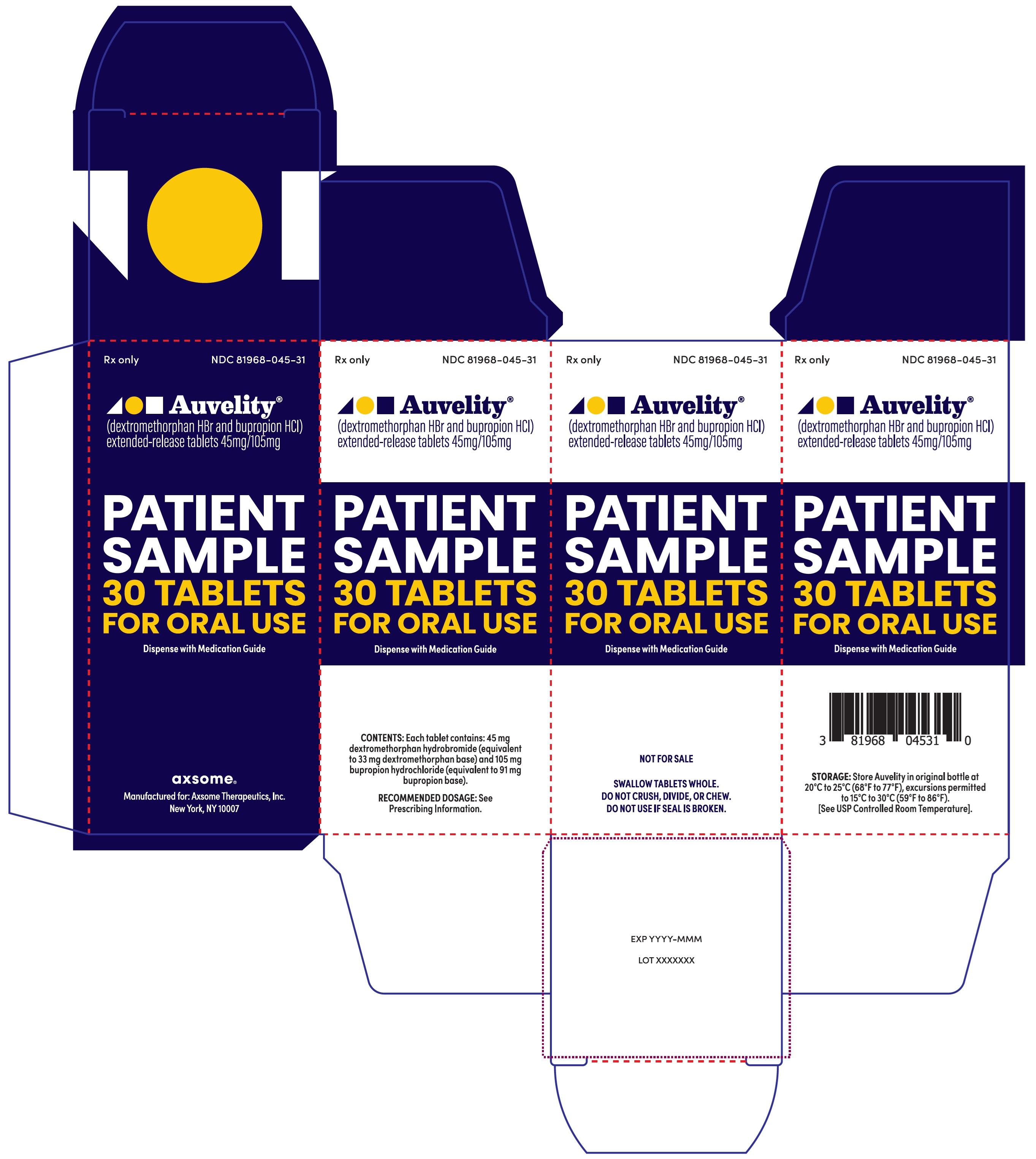
PRINCIPAL DISPLAY PANEL - NDC: 81968-045-31 - 30-count Physician Sample Carton LabelRx only - NDC 81968-045-31 - Auvelity® (dextromethorphan HBr and bupropion HCl) extended-release tablets 45mg/105mg - Patient Sample - 30 Tablets - For oral use - Dispense with Medication ...
-
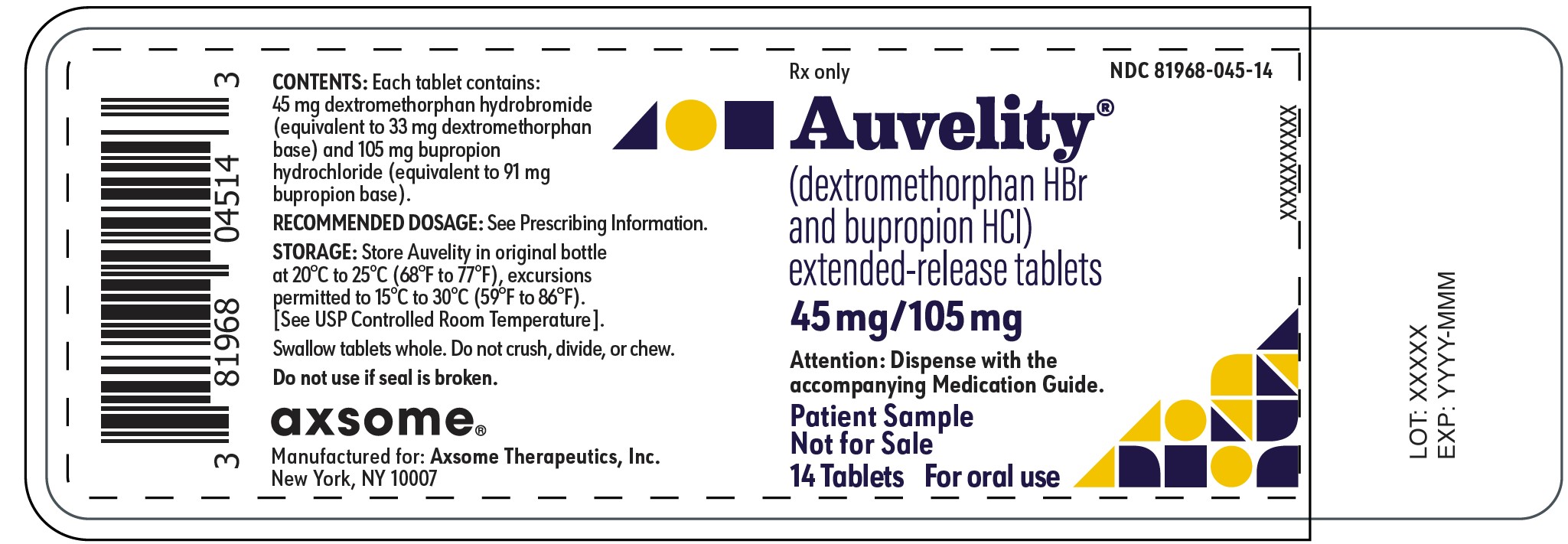
PRINCIPAL DISPLAY PANEL - NDC: 81968-045-14 - 14-count Physician Sample Bottle LabelRx only - NDC 81968-045-14 - Auvelity® (dextromethorphan HBr and bupropion HCl) extended-release tablets - 45mg/105mg - Attention: Dispense with the accompanying Medication Guide. Patient ...
-
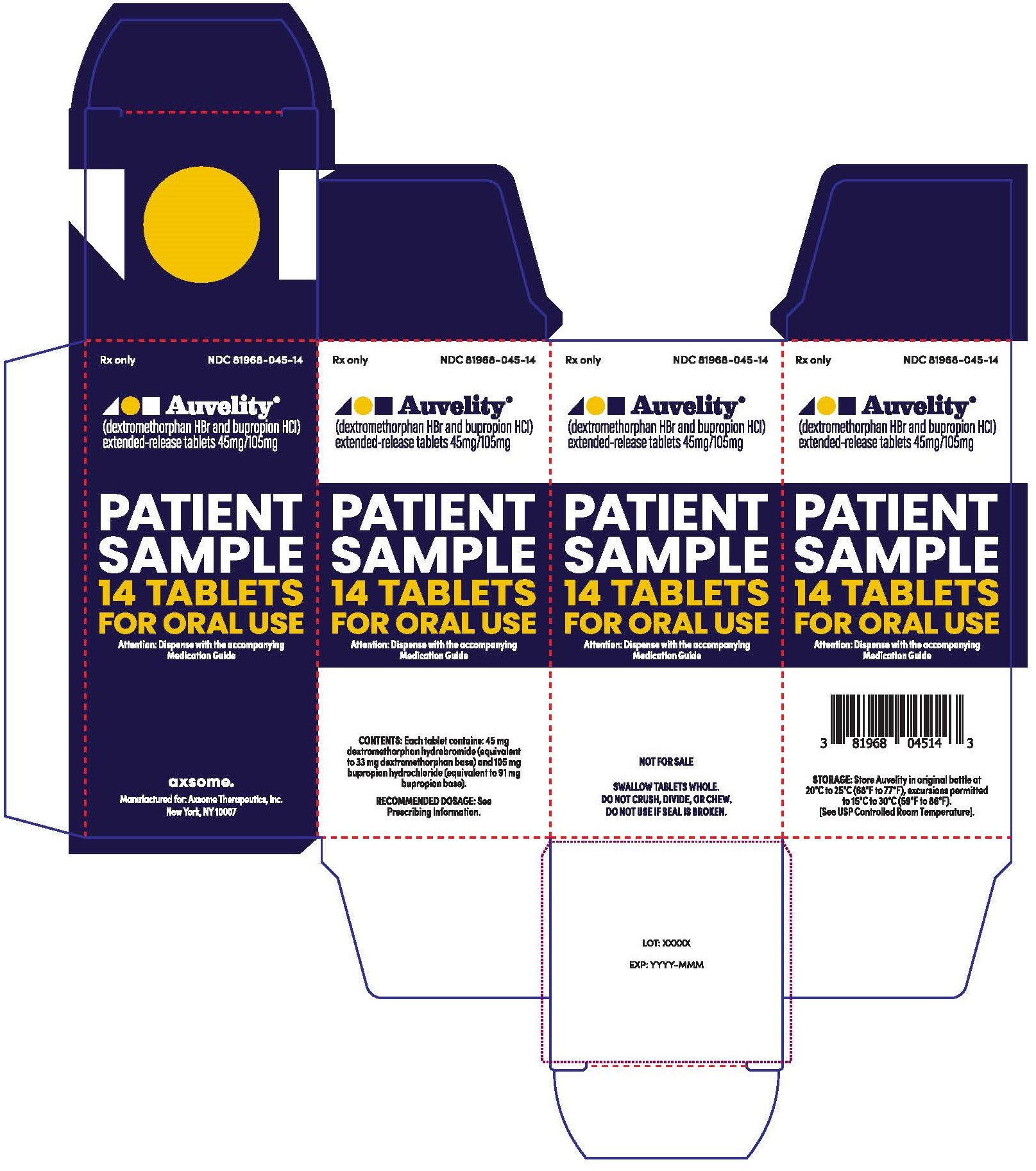
PRINCIPAL DISPLAY PANEL - NDC: 81968-045-14 - 14-count Physician Sample Carton LabelRx only - NDC 81968-045-31 - Auvelity® (dextromethorphan HBr and bupropion HCl) extended-release tablets 45mg/105mg - Patient Sample - 30 Tablets - For oral use - Dispense with Medication ...
-
INGREDIENTS AND APPEARANCEProduct Information