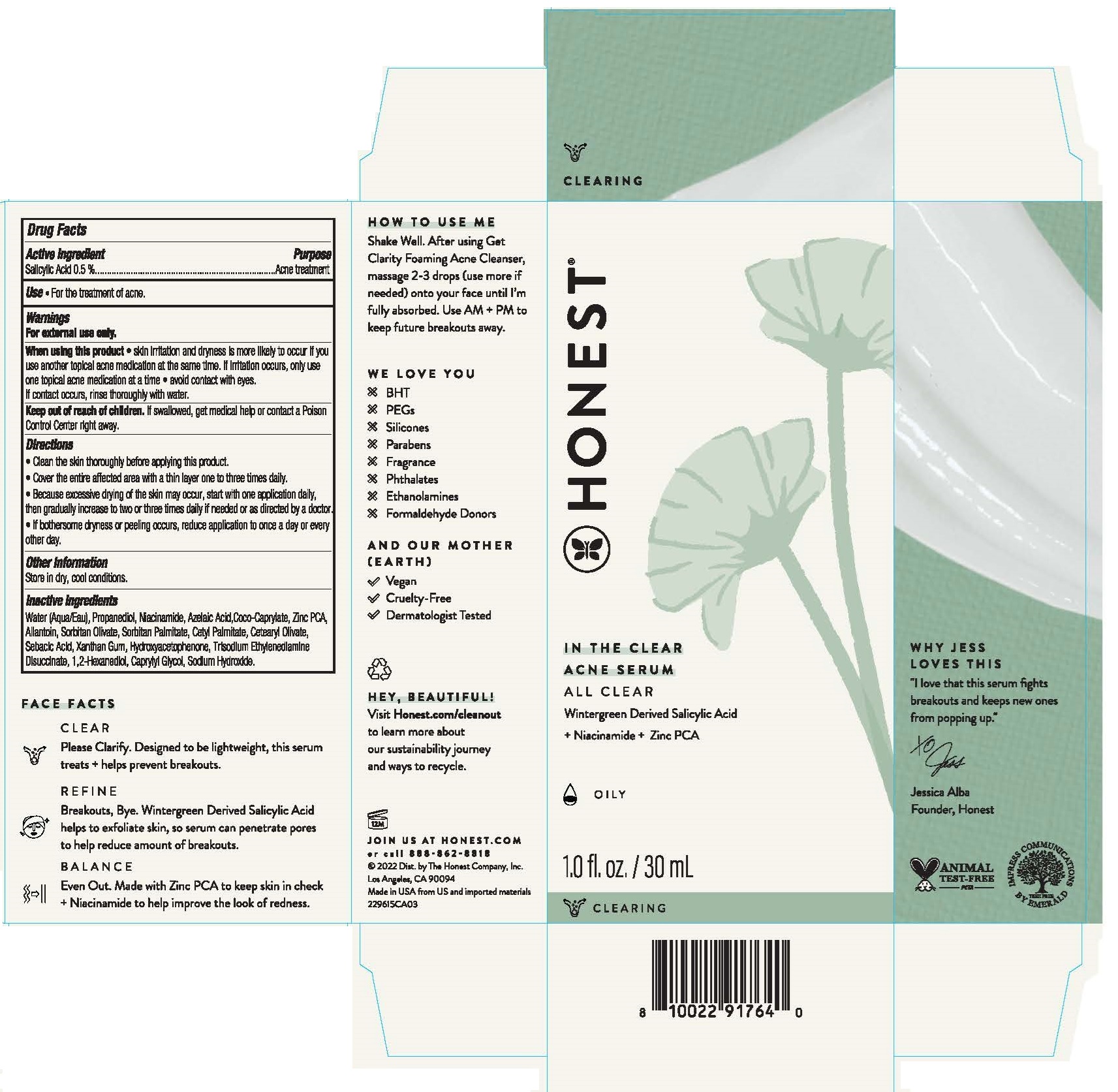
Label: IN THE CLEAR ACNE SERUM- salicylic acid liquid
- NDC Code(s): 69366-414-31
- Packager: The Honest Company, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 19, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
For external use only.
-
DOSAGE & ADMINISTRATION
Directions
• Clean the skin thoroughly before applying this product.
• Cover the entire affected area with a thin layer one to three times daily.
• Because excessive drying of the skin may occur, start with one applicaiton daily, then gradually increase to two or three times daily if needed or as direted by a doctor.
• If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
- SPL UNCLASSIFIED SECTION
-
INACTIVE INGREDIENT
Inactive ingredients
Water (Aqua/Eau), Propanediol, Niacinamide, Azelaic Acid, Coco-Caprylate, Zinc PCA, Allantoin, Sorbitan Olivate, Sorbitan Palmitate, Cetyl Palmitate, Cetearyl Olivate, Sebacic Acid, Xanthan Gum, Hydroxyacetophernone, Trisodium Ethylenedlamine Disuccinate, 1,2-Hexanediol, Caprylyl Glycol, Sodium Hydroxide.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
IN THE CLEAR ACNE SERUM
salicylic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69366-414 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 0.5 g in 100 mL Inactive Ingredients Ingredient Name Strength NIACINAMIDE (UNII: 25X51I8RD4) COCO-CAPRYLATE (UNII: 4828G836N6) CETEARYL OLIVATE (UNII: 58B69Q84JO) AZELAIC ACID (UNII: F2VW3D43YT) ZINC PIDOLATE (UNII: C32PQ86DH4) ALLANTOIN (UNII: 344S277G0Z) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) WATER (UNII: 059QF0KO0R) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) PROPANEDIOL (UNII: 5965N8W85T) SORBITAN OLIVATE (UNII: MDL271E3GR) SORBITAN MONOPALMITATE (UNII: 77K6Z421KU) CETYL PALMITATE (UNII: 5ZA2S6B08X) SEBACIC ACID (UNII: 97AN39ICTC) XANTHAN GUM (UNII: TTV12P4NEE) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69366-414-31 1 in 1 CARTON 05/01/2022 1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 05/01/2022 Labeler - The Honest Company, Inc (969962757)