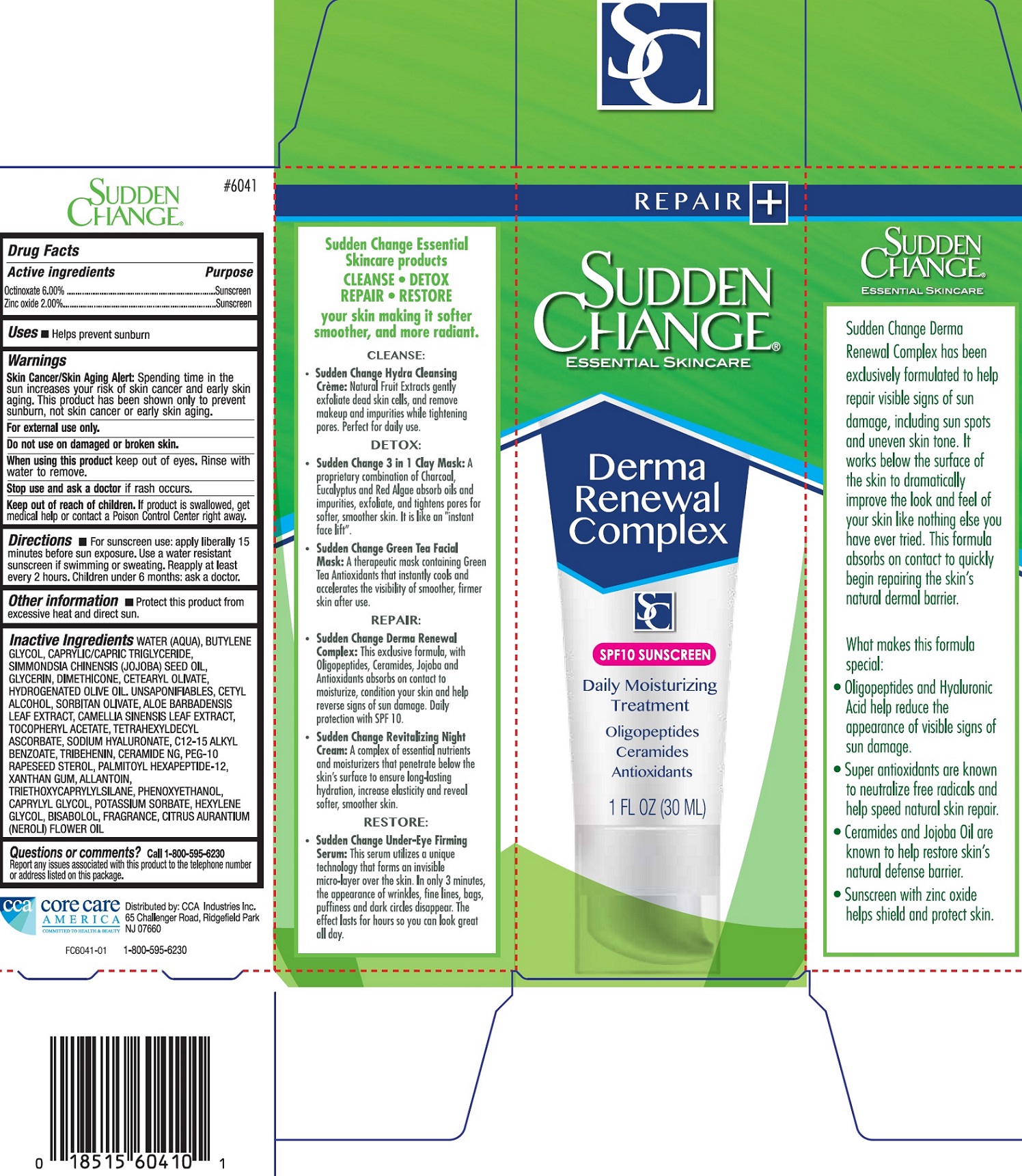
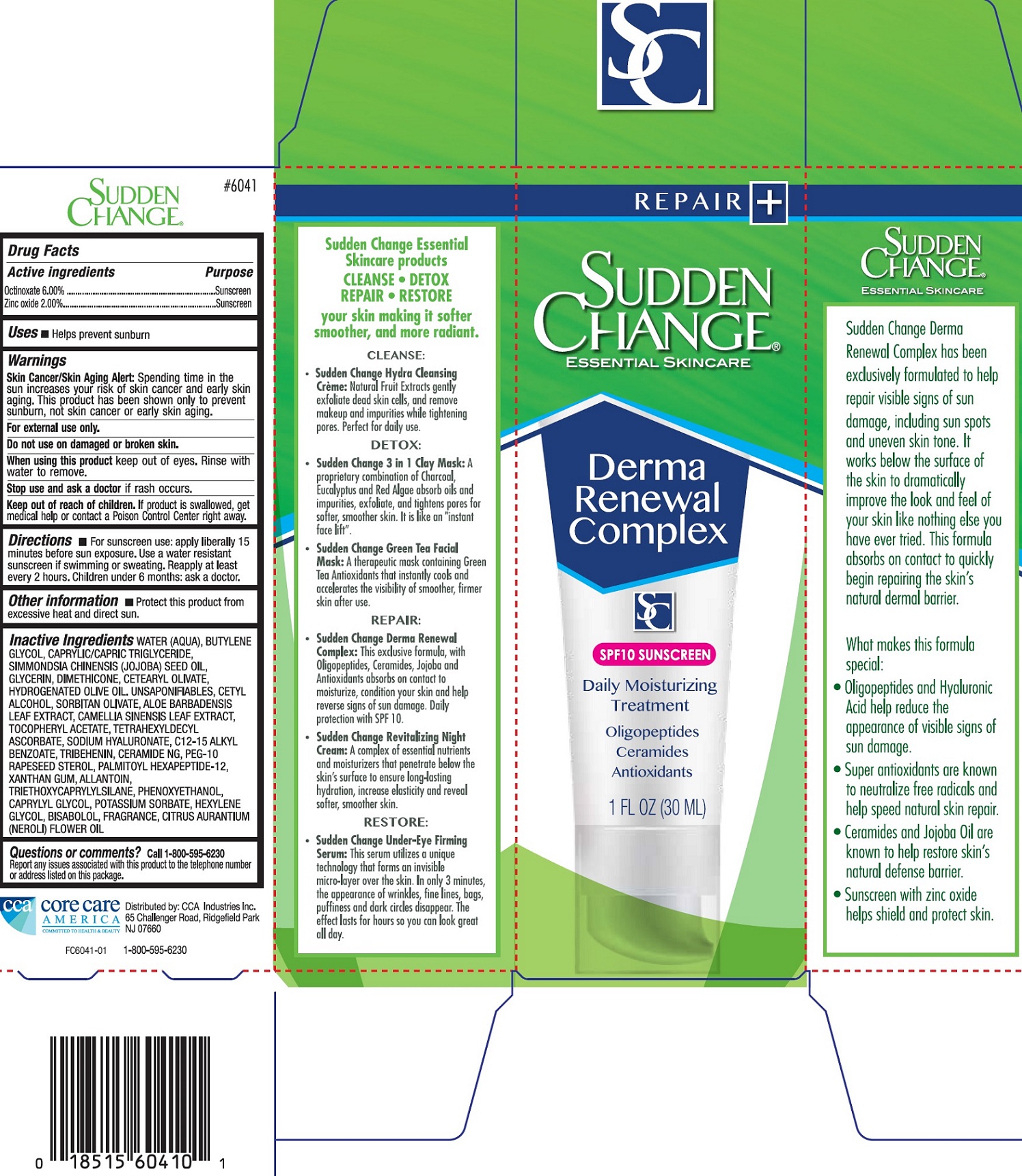
Label: SUDDEN CHANGE DERMA RENEWAL COMPLEX- octinoxate, zinc oxide cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 61543-0003-0 - Packager: CCA Industries Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 8, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredient
- Uses:
- Warnings
- Directions
- Other Information
-
Inactive Ingredients
WATER (AQUA), BUTYLENE GLYCOL, CAPRYLIC/CAPRIC TRIGLYCERIDE, SIMMONDSIA, CHINENSIS (JOJOBA) SEED OIL, GLYCERIN, DIMETHICONE, CETEARYL OLIVATE, HYDROGENATED OLIVE OIL, UNSAPONIFIABLES, CETYL ALCOHOL, SORBITAN OLIVATE, ALOE BARBADENSIS LEAF EXTRACT, CAMELLIA SINENSIS LEAF EXTRACT, TOCOPHERYL ACETATE, TETRAHEXYLDECYL ASCORBATE, SODIUM HYALURONATE, C12-15 ALKYL BENZOATE, TRIBEHENIN, CERAMIDE NG, PEG-10 RAPESEED STEROL, PALMITOYL HEXAPEPTIDE-12, XANTHAN GUM, ALLANTOIN, TRIETHOXYCAPRYLYLSILANE, PHENOXYETHANOL, CAPRYLYL GLYCOL, POTASSIUM SORBATE, HEXYLENE GLYCOL, BISABOLOL, FRAGRANCE, CITRUS AURANTIUM (NEROLI) FLOWER OIL
- Questions or Comments?
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
SUDDEN CHANGE DERMA RENEWAL COMPLEX
octinoxate, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61543-0003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 60 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) GLYCERIN (UNII: PDC6A3C0OX) DIMETHICONE (UNII: 92RU3N3Y1O) CETEARYL OLIVATE (UNII: 58B69Q84JO) HYDROGENATED OLIVE OIL (UNII: 53839415GI) OLEA EUROPAEA (OLIVE) OIL UNSAPONIFIABLES (UNII: XO45V955LT) CETYL ALCOHOL (UNII: 936JST6JCN) SORBITAN OLIVATE (UNII: MDL271E3GR) ALOE VERA LEAF (UNII: ZY81Z83H0X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) TRIBEHENIN (UNII: 8OC9U7TQZ0) CERAMIDE NG (UNII: C04977SRJ5) PEG-10 RAPESEED STEROL (UNII: 258O76T85M) PALMITOYL HEXAPEPTIDE-12 (UNII: HO4ZT5S86C) XANTHAN GUM (UNII: TTV12P4NEE) ALLANTOIN (UNII: 344S277G0Z) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PHENOXYETHANOL (UNII: HIE492ZZ3T) CAPRYLYL GLYCOL (UNII: 00YIU5438U) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) HEXYLENE GLYCOL (UNII: KEH0A3F75J) LEVOMENOL (UNII: 24WE03BX2T) CITRUS AURANTIUM FLOWER OIL (UNII: D4BGE91OXH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61543-0003-0 1 in 1 BOX 04/01/2018 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 04/01/2018 Labeler - CCA Industries Inc. (106771041)