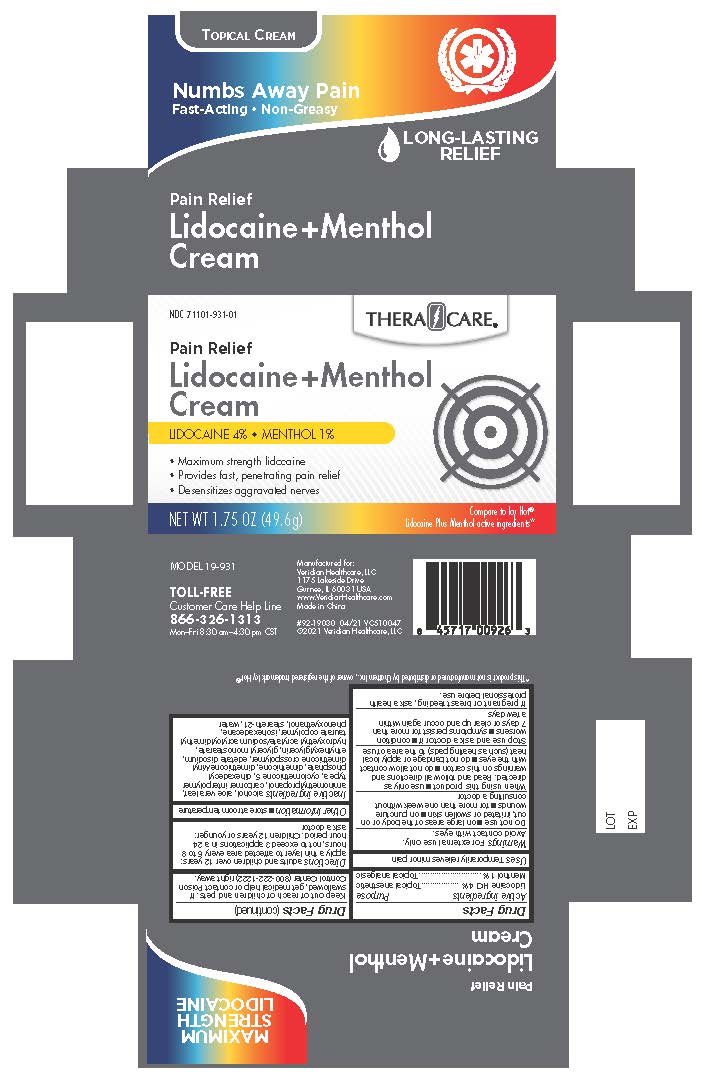
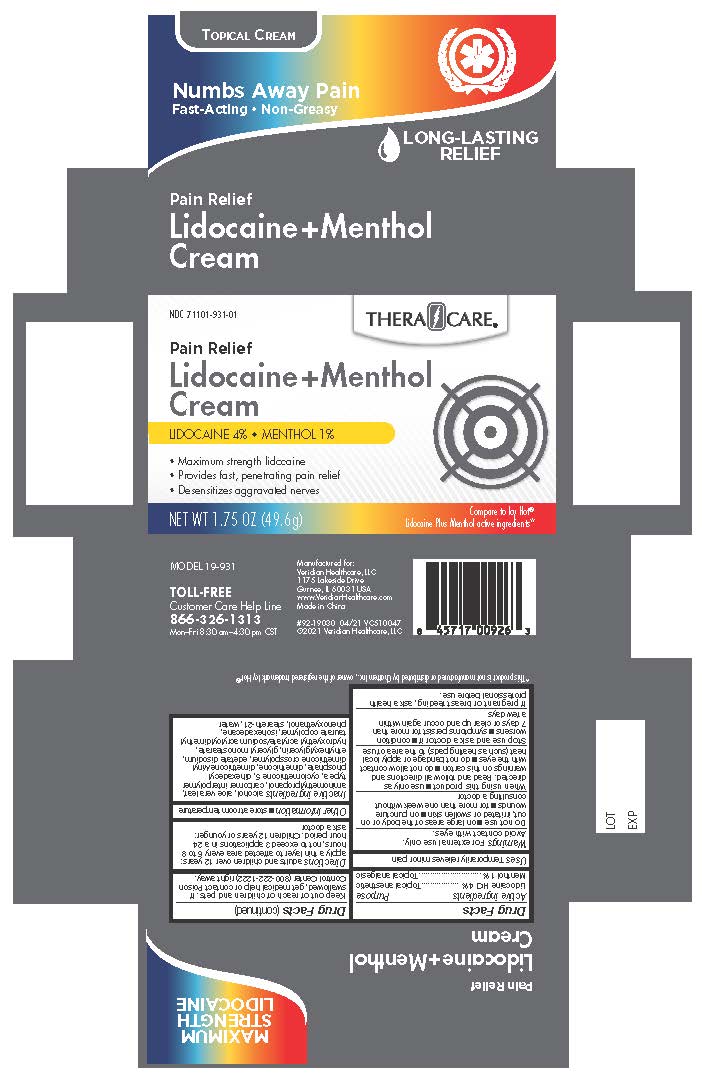
Label: THERACARE 4% LIDOCAINE AND 1% MENTHOL CREAM- menthol and lidocaine cream
- NDC Code(s): 71101-931-01
- Packager: Veridian Healthcare
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 21, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
-
Purposes
Lidocaine HCl 4%............................................................................................................................Topical anesthetic
Menthol 1%.......................................................................................................................................Topical analgesic
- Use
-
Warnings
For external use only. Avoid contact with eyes
Do not use
■ on large areas of the body or on cut, irritated or swollen skin
■ on puncture wounds
■ for more than one week without consulting a doctor
When using this product
■ use only as directed. Read and follow all directions and warnings on this carton.
■ do not allow contact with the eyes
■ do not bandage or apply local heat (such as heating pads) to the area of use
- Directions
-
Inactive ingredients
Alcohol, Aloe Vera Leaf, Aminomethylpropanol, Carbomer Interpolymer Type A, Cyclopentasiloxane 5, Dihexadecyl phosphate, Dimethicone, Dimethicone/Vinyl Dimethicone Crosspolymer, edetate disodium, Ethylhexylglycerin, Glyceryl monostearate, Hydroxyethyl Acrylate/ Sodium Acryloyldimethyl Taurate Copolymer, Isohexadecane, Phenoxyethanol, Steareth-21, Water
- PRINCIPAL DISPLAY PANEL TheraCare 4% Lidocaine & 1% Menthol Cream
-
INGREDIENTS AND APPEARANCE
THERACARE 4% LIDOCAINE AND 1% MENTHOL CREAM
menthol and lidocaine creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71101-931 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 4 g in 100 g MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 1 g in 100 g Inactive Ingredients Ingredient Name Strength STEARETH-21 (UNII: 53J3F32P58) DIMETHICONE (UNII: 92RU3N3Y1O) EDETATE DISODIUM (UNII: 7FLD91C86K) ALCOHOL (UNII: 3K9958V90M) PHENOXYETHANOL (UNII: HIE492ZZ3T) DIHEXADECYL PHOSPHATE (UNII: 2V6E5WN99N) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) ALOE VERA LEAF (UNII: ZY81Z83H0X) ISOHEXADECANE (UNII: 918X1OUF1E) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (45000 MPA.S AT 1%) (UNII: 86FQE96TZ4) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) WATER (UNII: 059QF0KO0R) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71101-931-01 1 in 1 CARTON 07/01/2021 1 49.6 g in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 07/01/2021 Labeler - Veridian Healthcare (830437997) Establishment Name Address ID/FEI Business Operations Nantong Health & Beyond Hygienic Products Inc. 421280161 manufacture(71101-931)